Using the molecular orbital model for a diatomic molecule, explain the different bond lengths for the ions
Question:
Using the molecular orbital model for a diatomic molecule, explain the different bond lengths for the ions of oxygen. Also state which ion is diamagnetic.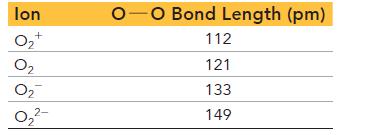
Transcribed Image Text:
lon O₂+ 0₂ 0₂ 0₂²- -O Bond Length (pm) 112 121 133 149
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The bond length of the O s...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The diatomic molecule OH exists in the gas phase. OH plays an important part in combustion reactions and is a reactive oxidizing agent in polluted air. The bond length and bond energy have been...
-
2 Prove that the irradiance of a harmonic EM-wave is given by, 1 = (c/2o)B,? Determine the average rate at which energy is transported per unit area by a plane wave having an amplitude of 15.0 V/m.
-
Molecular orbitals are most commonly delocalized throughout the molecule and exhibit distinct bonding or anti-bonding character. Loss of an electron from a specific molecular orbital from excitation...
-
In Exercises find the derivative of the function by the limit process. f(x) = x - 4x + 5
-
Tell whether each of the following actions will increase, decrease, or have no effect on total assets, total liabilities, and total stockholders equity: 1. Declaration of a stock dividend 2....
-
What is ABC inventory classification? How can this method be useful to a business?
-
Refer to the information in QS 13-4. Use that information for Tide Corporation to determine the 2016 and 2017 common-size percents for cost of goods sold using net sales as the base. Data From QS...
-
Tri-State Bank and Trust is considering giving Josef Company a loan. Before doing so, management decides that further discussions with Josefs accountant may be desirable. One area of particular...
-
= 1, 2, 3,.... Problem 3.32. Suppose (xi) 1 C R and xi xi+1 for all i Show that there is an x* = (-, 0] such that x converges to x*. That is {x} x*, though perhaps x* .
-
Sulfur dioxide is a reducing agent. When it is bubbled through an aqueous solution containing Br 2 , a red-colored solution, it reduces the bromine to colorless bromide ions and forms sulfuric acid....
-
Find the amount (in moles) of CC bonds that must be broken when 1.0 mole of C(g) is formed from C(diamond). Calculate the H of sublimation of diamond from the data in Appendix II, Table B. Then do...
-
Define comparability.
-
Compensation discrimination can be costly to organizations and as such, companies must work hard to ensure that their compensation practices are in alignment with legislation. Considering this please...
-
Question 13 - The objectives in developing a master schedule include the following except: The MS must balance the workload for a given company in terms of not only total capacity but also capacity...
-
How can a lack of empowerment affect customer service in a resort hotel?
-
Which power or combination of power styles would use to empower employees ethically? Support your answer.
-
For a new cleaning robot coming to the market: Human resources and job design. a) Recruit, motivate, and retain personnel with the required talent and skills (job description needed) b) Integral and...
-
The point of focus is the notion of "honor" in Europe during the 17th & 18th centuries. I would like some discussion of this element of European society especially as you read about Europes conquest...
-
Fred Farmer needs to prepare a balance sheet for his bank. He spent the day getting the following information. Fred needs your help to build a balance sheet and evaluate it. The information was...
-
Consider the square current loop, case 3 in Figure P20.57. It has an edge length L = 0.33 m and carries a current I = 7.5 A. The magnetic field B = 0.22 T is perpendicular to the plane of the loop....
-
Consider a square current loop (L = 25 cm) that carries a current I = 5.6 A (Fig. P20.59). A constant magnetic field B = 0.25 T makes an angle u 30 with the direction normal to the plane of the loop...
-
The plane of a circular current loop is oriented at a nonzero angle u relative to a magnetic field parallel to z (Fig. P20.60). If the current loop is free to rotate about an axis perpendicular to z,...
-
An array A contains N integers having values between 0 and n-1 (inclusive). The histogram of A is an array H of size n for which the entry i contains the number of occurences of element i in A. Here...
-
The code below reverses the order of items in a plain C++ array. Explain the logic briefly. Can the same approach be used with a singly-linked list? What about a doubly-linked list? Justify your...
-
Examine the following C++ program and give its output. (1 mark). #include #include #include using namespace std; void search vector & vec, int val) { } cout < < "searching for " < < val < < " ... ";...

Study smarter with the SolutionInn App


