Given the values of H rxn , and T, determine S rxn , and predict whether or
Question:
Given the values of ΔHrxn, and T, determine ΔSrxn, and predict whether or not each reaction is spontaneous.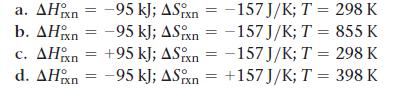
Transcribed Image Text:
a. AH n = -95 kJ; ASxn-157J/K; T = 298 K b. AH = -95 kJ; ASn = -157 J/K; T = 855 K c. AHxn = -157 J/K; T = 298 K +95 kJ; ASn = -95 kJ; ASixn -95 kJ; ASn = d. AH = +157J/K; T = 398 K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To determine the spontaneity of a reaction given the values of Hrxn and Twe can use the Gibbs f...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the change in Gibbs free energy for each of the sets of H rx n , S rxn , and T given in Problem 41. Predict whether or not each reaction is spontaneous at the temperature indicated. Problem...
-
Calculate the change in Gibbs free energy for each of the sets of H rx n , S rx n , and T given in Problem 42. Predict whether or not each reaction is spontaneous at the temperature indicated....
-
Given the values of H rxn , S rxn , and T, determine S univ and predict whether or not each reaction is spontaneous. a. AH n = +115 kJ; ASxn=-263 J/K; T = 298 K b. AH n = -115 kJ; ASn = +263 J/K; T...
-
Process compliance can be affected by: Multiple select question. organizational structure. maverick purchasing. organizational culture. information systems
-
As a country progresses from one economic stage to another, what in general are the marketing effects?
-
Hamilton, Inc., uses a job system of cost accounting. The data presented here relate to operations in its plant during January. Hamilton, Inc., has two production departments and one service...
-
In 1970, Rose Mary Knick purchased 90 acres of land in Scott Township, Lackawanna County, Pennsylvania. In 2008, another resident of Scott Township discovered documents that suggested that one of...
-
Vitalite, Inc., produces a number of products, including a body-wrap kit. Standard variable costs relating to a single kit are given below: During August, 500 kits were manufactured and sold....
-
No Excel solution required Derive a formula that calculates the present value of an annuity that pays $1376 pa quarterly in perpetuity given an annual effective rate of interest of 3.7%. What is the...
-
Calculate the free energy change for this reaction at 25 C. Is the reaction spontaneous? C3H8(g) + 5O(g) 5 O(g) 3 CO(g) + 4HO(g) AHin = -2217 kJ; ASixn 101.1 J/K rxn
-
A reaction has H rxn = -112 kJ and S rxn = 354 J/K. At what temperature is the change in entropy for the reaction equal to the change in entropy for the surroundings?
-
On January 1, 2010, Patrick Company purchased 100 percent of the outstanding voting stock of Shawn, Inc., for $1,000,000 in cash and other consideration. At the purchase date, Shawn had common stock...
-
Talent Acquisition and Interviews: Include appropriate citations and at least 3 references to support your response. Narrowing a pool of candidates can be difficult. The hiring manager must evaluate...
-
Prior to working on this discussion forum, read the article Organizational Life Cycles and Shifting Criteria of Effectiveness: Some Preliminary Evidence Download Organizational Life Cycles and...
-
1. Why has "Emotional Intelligence" transformed into a trending topic in project management? Please provide a holistic analysis to shed light on key factors that have contributed to the formation of...
-
In strategy development, once the vision, mission, values, and objectives are set, the next step is to identify strategic options. Once a strategic approach is determined, it is time to set up...
-
A firm's global operations function is focused on managing the development, production planning, sourcing, and distribution of the company's products. Consider an international firm with which you...
-
Discuss how the companys management team can manipulate its earnings results though the adoption of different accounting methods and the obligation of accounting professionals to be transparent to...
-
Orange juice producers are dismayed and puzzled. An economist told them that the reason the demand for orange juice fell is that a new technology allow tomato producers to pick ripe tomatoes more...
-
A long-throated flume is installed in a rectangular channel using design A from Table 14.5. Compute the discharge for a head of 0.35 ft.
-
Figure P.10.30 is a computer-generated Fraunhofer irradiance distribution. Describe the aperture that would give rise to such a pattern and give your reasoning in detail. Figure P.10.30
-
Figure P.10.31 is the electric-field distribution in the far field for a hole of some sort in an opaque screen. Describe the aperture that would give rise to such a pattern and give your reasoning in...
-
Select one individual and one organizational barrier to innovation. What criteria would you use to determine how these barriers prevent innovation? Support the importance of knowledgeable management...
-
Cast Iron Fabrication allocates manufacturing overhead to each job using departmental overhead rates. The company's operations are divided into a casting department and a finishing department. The...
-
Even without documentation, couldn't the staff testify much he drank and when? Explain your answer. as to how

Study smarter with the SolutionInn App


