How many grams of water form when 1.24 L of H 2 gas at STP completely reacts
Question:
How many grams of water form when 1.24 L of H2 gas at STP completely reacts with O2?![]()
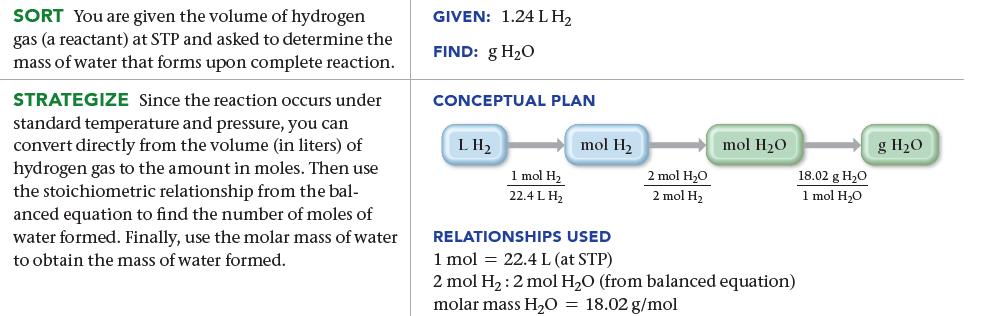
Transcribed Image Text:
2 H₂(g) + O2(8) 2 H₂O(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
124 LH X 1 mol ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
How many grams of water form when 2.41 L of oxygen gas at STP completely react with an appropriate amount of H 2 ? 2 H 2 (g) + O 2 (g) 2 H2O(g)
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
The following are the financial statements of Swifty Corporation. Swifty Corporation Comparative Balance Sheets December 31 Assets 2019 2018 Cash $37,200 $19,700 Accounts receivable 33,000 18,400...
-
Lacoste t-shirts come with an average price of $ 120 a piece, at their factory outlet with a std. deviation of $ 17. But at the Seasonal Sale (Discount) outlets of these t- shirts, it was also...
-
Analysts claim that businesses can increase sales on the Internet, but not profits. What evidence does this chapter provide to support or refute this claim? Discuss.
-
Suppose a file system is organized like the DOS file system and the device index contains 64K pointers. Explain how the file manager could be designed to use the 64K pointers to reference every...
-
In 2014, political consulting firm Cambridge Analytica developed an app designed to create digital profiles of individuals via their information. Cambridge Analytica collected the data by inviting...
-
The Silver Star Bicycle Company will be manufacturing both mens and womens models for its Easy-Pedal 10-speed bicycles during the next two months. Management wants to develop a production schedule...
-
Discuss similarities and differences of A. MongoDB, B. Amazon (AWS) database management system to relational database management systems. Indicate the benefits or disadvantages you associate with the...
-
Define molar volume and list its value for a gas at STP.
-
A sample of Xe takes 75 seconds to effuse out of a container. An unknown gas takes 37 seconds to effuse out of the identical container under identical conditions. What is the most likely identity of...
-
You have 1.5 moles of pure water and 1 mole of CO, both at 25C and 1 bar. You want to mix them together to make carbon dioxide by the following gas phase reaction at 500 K: CO + H 2 O CO 2 + H 2...
-
Explain organizing for safety.
-
Many banks and similar lending institutions require that the chief executive officer (CEO) of small corporations (which usually are owned by the CEO and a few relatives) cosign any loan made to the...
-
You decide to sell a house that you bought five years ago with the purpose of renting it. Its purchase price was $300,000, and now its market value is about $500,000. How much should you expect to...
-
What factors influence the rate assigned to a contractor for Workmens Compensation insurance?
-
What factors should motivate a contractor to have a safe operation and a good safety program?
-
In 2010, the Lawrence Company spends $4 million drilling oil wells. Sixty percent of the drilling is successful and results in commercial quantities of oil being found. Required 1. How much drilling...
-
Cleaning Service Company's Trial Balance on December 31, 2020 is as follows: Account name Debit Credit Cash 700 Supplies Pre-paid insurance Pre-paid office rent Equipment Accumulated depreciation -...
-
Determine the moments at the supports A and C, then draw the moment diagram. Assume joint B is a roller. EI is constant. 25 kN 15 kN/m A. - 4 m- - 3 m -3 m-
-
Determine the moments at A, B, and C, then draw the moment diagram for the beam. The moment of inertia of each span is indicated in the figure. Assume the support at B is a roller and A and C are...
-
Determine the moments at A, B, and C and then draw the moment diagram. EI is constant. Assume the support at B is a roller and A and C are fixed. 3 k 3k B. |- 3 ft--3 ft -3 ft -- -10 ft- 10 ft
-
Sleep Tight, Inc., manufactures bedding sets. The budgeted production is for 48,400 comforters this year. Each comforter requires 1.5 hours to cut and sew the material. The cost of cutting and sewing...
-
Kubin Company's relevant range of production is 27,000 to 29,000 units. When it produces and sells 28,000 units, its average costs per unit are as follows: Average Cost per Unit Direct materials $...
-
Serendipity Sound, Incorporated, manufactures and sells compact discs. Price and cost data are as follows: Selling price per unit (package of two CDs) Variable costs per unit: Direct material Direct...

Study smarter with the SolutionInn App


