Hydrogen can be extracted from natural gas according to the reaction: An 85.0-L reaction container initially contains
Question:
Hydrogen can be extracted from natural gas according to the reaction: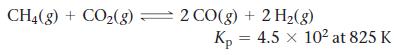
An 85.0-L reaction container initially contains 22.3 kg of CH4 and 55.4 kg of CO2 at 825 K. Assuming ideal gas behavior, calculate the mass of H2 (in g) present in the reaction mixture at equilibrium.
What is the percent yield of the reaction under these conditions?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





