In each reaction, identify the BrnstedLowry acid, the BrnstedLowry base, the conjugate acid, and the conjugate base.
Question:
In each reaction, identify the Brønsted–Lowry acid, the Brønsted–Lowry base, the conjugate acid, and the conjugate base.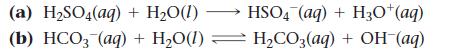
Transcribed Image Text:
(a) H₂SO4(aq) + H₂O(1) (b) HCO3(aq) + H₂O(1) HSO4 (aq) + H3O+ (aq) H₂CO3(aq) + OH(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
a Because HSO4 donates a proton to HO in this reaction it is the acid pr...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
An investor wishes to analyse the effects of different compounding frequencies Suppose 1000 is invested for 1 year at an interest rate of 5 per annum compounded Assume there are 365 days in 1 year
-
If 100 mol methanol and 100 mol acetic acid are fed to a batch reactor and allowed to come to equilibrium. How many moles of each component will be present in the product stream?
-
The conjugate base of diethyl malonate can serve as a nucleophile to attack a wide range of electrophiles. Identify the product that is formed when the conjugate base of diethyl malonate reacts with...
-
Describe three ways in which a gradual increase in an extracellular signal can be sharpened by the target cell to produce an abrupt or nearly all-or none response.
-
Define the three processes of the process value chain.
-
A block diagram of a closed-loop system is shown in figure.(a) Derive a closed-loop transfer function for disturbance changes, Y(s)/Ds).(b) For the following transfer functions, what values of Kc...
-
In 1940, the family of Thomas Back entered into an oil-and-gas lease with the Inland Gas Corporation. The lease held that Inland would pay to Backs family 12 cents per thousand cubic feet of gas...
-
The Bruhaha Brewery is planning to expand internationally. The company has identified six critical location factors and their relative weights. The scores for each of the three potential sites are...
-
John just sold the home where he's lived for eight years and made a nice profit. Which of these statements about the profit he made is FALSE? Unset starred question He can include depreciation costs...
-
Which property is not associated with an acid? (a) Dissolves metals (b) Turns blue litmus red (c) Has a bitter taste
-
Define each of the following with complete sentences, and provide an example chemical equation: an Arrhenius acid, a BrnstedLowry base, and a Lewis acid.
-
Given the program below, what is the output if the user enters 3 in response to the prompt? import java.util. Scanner; public class Athletes { public static void main(String[] args) { Scanner stdIn...
-
In recent decades, the Federal Reserve has transitioned from an intentionally vague and bureaucratic communication style to a more direct and transparent approach. What is the impact on inflation...
-
What dimensions of the Ignatian ethos resonate with your approach to leading organizational change during these turbulent times?
-
What happens to the amount Americans pay for imported goods if the price of the U.S. dollar depreciates? Will Americans import more or fewer goods if the U.S. dollar depreciates?
-
How can cultural congruence be achieved in an organization since there is a likelihood of conflict among national, organizational, and individual cultural orientations?
-
An adjustment needs to be made to the unrealized profit included in the inventory balance to avoid the understatement of group profits and the overstatement of group assets. True/False
-
Shown here are annual financial data at December 31, 2013, taken from two different companies. Required 1. Compute the cost of goods sold section of the income statement at December 31, 2013, for...
-
Read the case study Richter: Information Technology at Hungarys Largest Pharma and answer the following question: How does the organization ensure the accuracy of the data it stores?
-
A device used to measure the radius of curvature of the cornea of the eye is called a keratometer. This is useful information when fitting contact lenses. In effect, an illuminated object is placed a...
-
An object is located at a distance s 0 from a spherical mirror of radius R.Show that the resulting image will be magnified by an amount R MT = 2s, + R
-
Design a little dentists mirror to be fixed at the end of a shaft for use in the mouth of some happy soul. The requirements are (1) that the image be erect as seen by the dentist and (2) that when...
-
The following transactions for Bargain Tire, Inc., occurred during November. (Click the icon to view the transactions.) Requirements 1. Journalize the transactions on the books of Bargain Tire, Inc....
-
Layout References Malings Problem #1 Points: 25 Review Help Use the information in Part I for LeBron James Company to complete a Cost of Goods Sold Statement for the year ended 2025. All reports...
-
Human Resources in the retail sector is undergoing significant change and transformation and the following areas are key to driving the transformation. Please explain your understanding of the policy...

Study smarter with the SolutionInn App


