Magnesium metal reacts with hydrochloric acid according to the balanced equation: In an experiment to determine the
Question:
Magnesium metal reacts with hydrochloric acid according to the balanced equation:![]()
In an experiment to determine the enthalpy change for this reaction, 0.158 g of Mg metal is combined with enough HCl to make 100.0 mL of solution in a coffee-cup calorimeter. The HCl is sufficiently concentrated so that the Mg completely reacts. The temperature of the solution rises from 25.6 °C to 32.8 °C as a result of the reaction. Find ΔHrxn for the reaction as written. Use 1.00 g/mL as the density of the solution and Cs, soln = 4.18 J/g · °C as the specific heat capacity of the solution.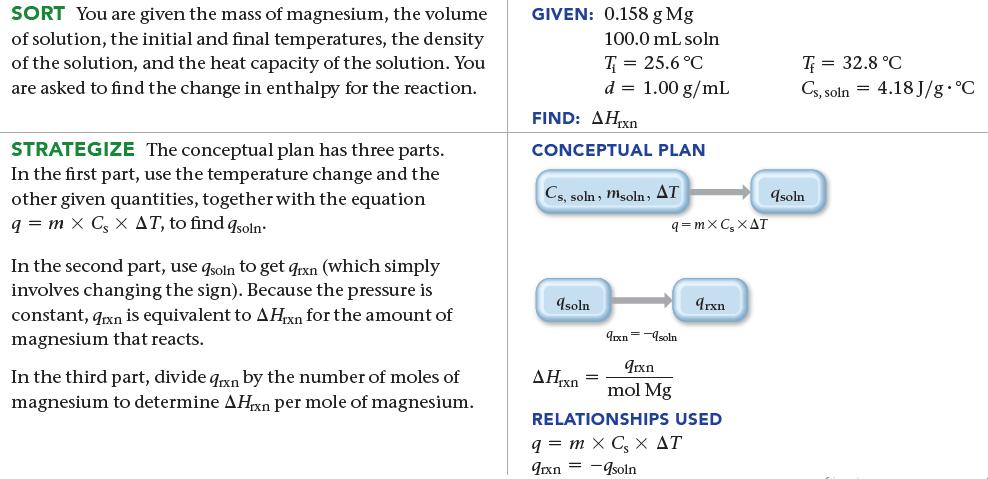
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





