Nitrogen and hydrogen react to form ammonia according to the following equation: Consider the following representations of
Question:
Nitrogen and hydrogen react to form ammonia according to the following equation:![]()
Consider the following representations of the initial mixture of reactants and the resulting mixture after the reaction has been allowed to react for some time: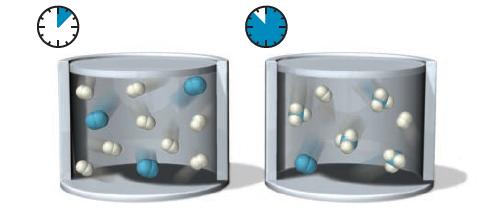
If the volume is kept constant, and nothing is added to the reaction mixture, what happens to the total pressure during the course of the reaction?
(a) The pressure increases.
(b) The pressure decreases.
(c) The pressure does not change.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





