Silver sulfate dissolves in water according to the reaction: A 1.5-L solution contains 6.55 g of dissolved
Question:
Silver sulfate dissolves in water according to the reaction: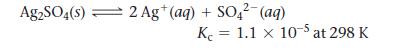
A 1.5-L solution contains 6.55 g of dissolved silver sulfate. If additional solid silver sulfate is added to the solution, will it dissolve?
Transcribed Image Text:
Ag2SO4(s) 2 Ag+ (aq) + SO42- (aq) K 1.1 x 10-5 at 298 K =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
Additi...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
A sample consisting of 22.7 g of a nongaseous, unstable compound X is placed inside a metal cylinder with a radius of 8.00 cm, and a piston is carefully placed on the surface of the compound so that,...
-
When a pure substance is placed in contact with water, there are three possible outcomes. The substance may do nothing that is, the substance does not dissolve and no visible change takes place. The...
-
To the right is the graph of the position of the object versus time: Which of graphs below correctly shows the object's velocity versus time? velocity (m/s) velocity (m/s) 1.5 1 0.5 -0.5 -1 -1.5 15...
-
Jonfran Company manufactures three different models of paper shredders including the waste container, which serves as the base. While the shredder heads are different for all three models, the waste...
-
Under what circumstances do page faults occur? Describe the actions taken by the operating system when a page fault occurs.
-
What is the System Definition Phase, when does it start, and when does it end?
-
The current balance sheet of J. J. Arvesen Company contains the following major sections: A. Current assets B. Long-term investments C. Property, plant, and equipment D. Intangible assets E. Other...
-
Does the private security employee still have the equivalent power of a private citizen? Should they? Why or why not
-
Nitrogen dioxide dimerizes according to the reaction: A 2.25-L container contains 0.055 mol of NO 2 and 0.082 mol of N 2 O 4 at 298 K. Is the reaction at equilibrium? If not, in what direction will...
-
Consider the reaction: A reaction mixture contains 0.112 atm of H 2 , 0.055 atm of S 2 , and 0.445 atm of H 2 S. Is the reaction mixture at equilibrium? If not, in what direction will the reaction...
-
What kind of code is placed in a script element?
-
A positively charged particle Q 1 = + 4 5 nC is held at fixed position. A second charge Q 2 of mass m = 3 . 5 mu g is located a distance d = 3 5 cm directly above charge Q 1 . The net force on Q 2 is...
-
You are flying your glider around your local airport for fun one late Saturday afternoon in July. The convective currents are great that day. You fly for about 1.5 hours enjoying a perfect day for...
-
Ren, a sales representative of an Electric Supply Inc made a demo for a new high-speed Auto[1]analyzer for Glen, the owner of a car repair service shop. The price of the Auto-analyzer was $3,500.00....
-
Please watch the following videos and answer the questions that follow. Michael Metcalfe: "A provocative way to finance the fight against climate change" What promise was made at the Copenhagen...
-
According to Governance, Risk & Compliance (2023), Jessica Fountaine, a woman from Florida got arrested on suspicion of fraud and third-degree grand theft in connection with a resident of Brandon...
-
When a balance sheet reports a substantial dollar amount for goodwill, discuss what we should be concerned with in our analysis.
-
A spacecraft has left the earth and is moving toward Mars. An observer on the earth finds that, relative to measurements made when the spacecraft was at rest, its a. length is shorter b. KE is less...
-
A bartender slides a mug of root beer with mass m = 2.6 kg down a bar top of length L = 2.0 m to an inattentive patron who lets the mug fall a height h = 1.1 m to the floor. The bar top (Fig. P4.72)...
-
Consider once again the swimmer in Example 4.7. Assume she can swim at a velocity of 0.30 m/s and the river is 15 m wide. She needs to get across the river as quickly as possible. (a) What direction...
-
You are a serious basketball player and want to use physics to improve your free-throw shooting. Do an approximate calculation of the minimum speed the ball must have to travel from your hand to the...
-
Brown Company issued $ 1 0 0 million of its 7 % bonds on April 1 , 2 0 2 4 at 8 5 ( $ 8 5 million ) plus accrued interest. The bonds are dated January 1 , 2 0 2 4 and have an effective interest rate...
-
Envelope Company began operations this month mass-producing pull-and-seal envelopes. During the month of July, it completed 25,000 units and has 20,000 units that are 65% complete. It had product...
-
The balance sheets of the companies immediately after the acquisition showed the amount Problem Company & 1 3 0 , 0 0 0 7 0 , 0 0 0 2 1 0 , 0 0 0 9 0 , 0 0 0 7 0 , 0 0 0 4 0 , 0 0 0 ) Investment...

Study smarter with the SolutionInn App


