The atmosphere slowly oxidizes hydrocarbons in a number of steps that eventually convert the hydrocarbon into carbon
Question:
The atmosphere slowly oxidizes hydrocarbons in a number of steps that eventually convert the hydrocarbon into carbon dioxide and water. Part of the process for methane gas is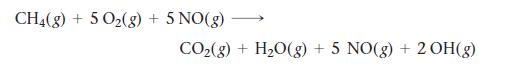
Suppose that an atmospheric chemist combines 155 mL of methane at STP, 885 mL of oxygen at STP, and 55.5 mL of NO at STP in a 2.0 L flask. The flask is allowed to stand for several weeks at 275 K. If the reaction reaches 90.0% of completion (90.0% of the limiting reactant is consumed), what is the partial pressure of each of the reactants and products in the flask at 275 K?
What is the total pressure in the flask?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





