The results of a molecular orbital calculation for H 2 O are shown here. Examine each of
Question:
The results of a molecular orbital calculation for H2O are shown here. Examine each of the orbitals and classify them as bonding, antibonding, or nonbonding. Assign the correct number of electrons to the energy diagram. According to this energy diagram, is H2O stable? Explain.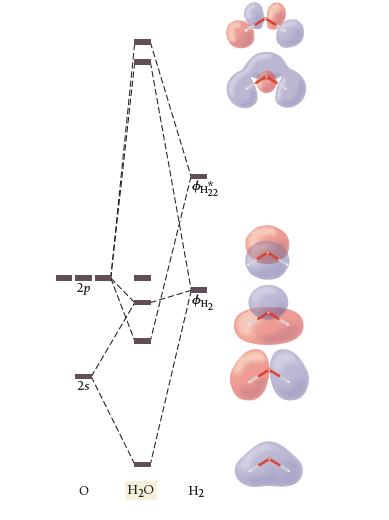
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





