cis-2-Butene isomerizes to trans-2-butene via the reaction shown here. a. If isomerization requires breaking the p bond,
Question:
cis-2-Butene isomerizes to trans-2-butene via the reaction shown here.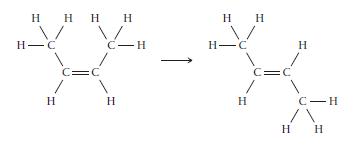
a. If isomerization requires breaking the p bond, what minimum energy is required for isomerization in J/mol? In J/molecule?
b. If the energy for isomerization came from light, what minimum frequency of light would be required? In what portion of the electromagnetic spectrum does this frequency lie?
Transcribed Image Text:
H Н Н Н Н н н H H-C H =C с-н Н Н H H-C н- 1 Н Н Н C Н C-H н н нн
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a To calculate the minimum energy required for isomerizationwe need to know the bond energy of the b...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The reaction shown here was performed with an iridium catalyst, both in supercritical CO2 (scCO2) and in the chlorinated solvent CH2Cl2. The kinetic data for the reaction in both solvents are plotted...
-
The energy of a vibrating molecule is quantized much like the energy of an electron in the hydrogen atom. The energy levels of a vibrating molecule are given by the equation: where n is a quantum...
-
The first LEDs were made from GaAs, which has a band gap of 1.43 eV. What wavelength of light would be emitted from an LED made from GaAs? What region of the electromagnetic spectrum does this light...
-
Gems Co. uses the indirect method to prepare its statement of cash flows. The following comparative statement of financial position for 2021 and 2022 are presented: At December 31 2022 2021 Property,...
-
Worldwide Travel Service has made an investment in certain equipment that cost the company $307,100. The equipment is expected to generate cash inflows of $50,000 each year. Required: How many years...
-
The 2007 Intel report can be found at the following Web site: www.prenhall .com/ fraser. Review the quality of financial reporting for Intel. Write a summary explaining whether the quality is good or...
-
How can computers be used to assist in the litigation process?
-
Myles Etter and Crystal Santori are partners who share in the income equally and have capital balances of $210,000 and $62,500, respectively. Etter, with the consent of Santori, sells one-third of...
-
Use one real world example to show me that we can create scarcity. ( 25-50 words). Sample answer Time-Limited scarcity: in time-limited offers, we need to decide before a set deadline - this adds a...
-
The results of a molecular orbital calculation for H 2 O are shown here. Examine each of the orbitals and classify them as bonding, antibonding, or nonbonding. Assign the correct number of electrons...
-
In VSEPR theory, which uses the Lewis model to determine molecular geometry, the trend of decreasing bond angles in CH 4 , NH 3 , and H 2 O is accounted for by the greater repulsion of lone pair...
-
A cubical Gaussian surface \(30 \mathrm{~mm}\) on each side is centered on a particle that carries a charge of \(+3.0 \mu \mathrm{C}\). (a) If you draw a field line diagram with four field lines per...
-
The Doley Company has planned the following sales for the next three months: Budgeted sales Jan $40,000 Feb $50,000 Mar $70,000 Sales are made 20% for cash and 80% on account. From experience, the...
-
The traditional Banking and Financial Service Industry within the Caribbean should fully integrate with emerging blockchain technologies to improve security and transparency and reduce exposure to...
-
Now that the SEC has approved a Bitcoin ETF based on CME Bitcoin Futures symbol BTIO: Do you think it is more likely or less likely that they will approve an ETF in the near future based on the spot...
-
In addition to the Earth s magnetic field, list some other sources of the ambient magnetic field in the lab. Which source do you think provides the largest contribution to the overall magnetic field?
-
According to the paper, is city compactness a productive amenity or an unproductive amenity? Explain why. Recall that a productive amenity is one which lowers costs for the firm. An unproductive...
-
Use your knowledge of balance sheets to fill in the amounts missing in the text. S 10,000 Cash Accounts receivable Inventorv Accounts pavable Notes payable Total current liabilities Long-term debt...
-
A routine activity such as pumping gasoline can be related to many of the concepts studied in this text. Suppose that premium unleaded costs $3.75 per gal. Work Exercises in order. Use the...
-
The 2-Mg concrete pipe has a center of mass at point G. If it is suspended from cables AB and AC, determine the average normal stress in the cables. The diameters of AB and AC are 12 mm and 10 mm,...
-
The 2-Mg concrete pipe has a center of mass at point G. If it is suspended from cables AB and AC, determine the diameter of cable AB so that the average normal stress in this cable is the same as in...
-
The pier is made of material having a specific weight g. If it has a square cross section, determine its width w as a function of z so that the average normal stress in the pier remains constant. The...
-
A firm's total cost and total revenue functions are as follows: () = 100 2 ()=2352+36+4 (a) Write an expression for the profit function (as a function ofQ) (5%) (b) Determine the optimal quantity,Q*,...
-
Hydrogen deuterium nuclei each have one proton with a charge of + 1 . 6 0 2 * 1 0 - 1 9 Coulombs. For nuclear fusion to occur between two hydrogen deuterium nuclei; the two nuclei must be brought...
-
Review your results below and play again with one of the other scenarios to test and develop your skills further. Real GDP Growth High Unemployment Rate Low Approval Rating Feedback from Policy...

Study smarter with the SolutionInn App


