The standard enthalpy of formation for glucose [C 6 H 12 O 6 (s)] is -1273.3 kJ/mol.
Question:
The standard enthalpy of formation for glucose [C6H12O6(s)] is -1273.3 kJ/mol. What is the correct formation equation corresponding to this ΔH°f?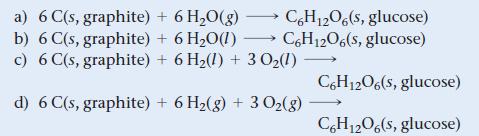
Transcribed Image Text:
a) 6 C(s, graphite) b) 6 C(s, graphite) + 6H₂O(g) →C6H12O6(s, glucose) + 6H₂O(1)→ C6H12O6(s, glucose) c) 6 C(s, graphite) + 6 H₂(1) + 3 0₂ (1) d) 6 C(s, graphite) + 6H₂(g) + 3 O₂(g) C6H1206(s, glucose) C6H1206(s, glucose)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
d 6 Cs g...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The standard enthalpy of formation of the metallocene bis (benzene) chromium was measured in a calorimeter. It was found for the reaction Cr (C6H6)2(s) Cr(s) + 2 C6H6 (g) that Uo (583 K) = +8.0 kJ...
-
White phosphorus exists as P 4 molecules with phosphorus atoms at the corners of a tetrahedron. (a) Elemental phosphorus reacts with Cl 2 to form PCl 3 . Write a balanced chemical equation for this...
-
The average energy output of a good grade of coal is 2.6 10 7 kJ/ton. Fission of 1 mol of 235 U releases 2.1 10 10 kJ. Find the number of tons of coal needed to produce the same energy as 1 lb of...
-
A six-lane freeway (three lanes in each direction) in a scenic area has a measured free-flow speed of 88.5 km/h. The peak-hour factor is 0.80, and there are 8% large trucks and buses and 6%...
-
The questions in this exercise are based on Toll Brothers, Inc. one of the largest home builders in the United States. To answer the questions, you will need to download Toll Brothers 2004 annual...
-
If a rifle fires a bullet straight up with a muzzle speed of 300 m/s, how high will the bullet rise?
-
Teddys daily budget constraint is shown in the following chart. Teddys employer pays him a base wage rate plus overtime if he works more than the standard hours. What is Teddys daily nonlabor income?...
-
Beachfront property owners of the Village of Eden requested a seawall be constructed to protect their beach. The seawall was financed through a note payable, which was to be repaid from taxes raised...
-
Transform following Relational model into ER Mode id Author name dob AuthorContact id contact Manager writes authorid bookid Book bookid title edition libid memberld location opening Time...
-
Which fuel is not a fossil fuel? a) Coal b) Hydrogen c) Natural gas d) Petroleum
-
What is heat? Explain the difference between heat and temperature.
-
Re-examine the X = 17.50 call for AMR in the previous exercise. a. Is the call correctly priced? b. What price would be necessary for this call in order for the implied volatility to be 60%?
-
Define a call options exercise value. Why is the actual market price of a call option usually above its exercise value?
-
What lessons can be learned from the quantitative risk assessment of prospective payment and capitation contracts?
-
Which one is most important to healthcare providers? Explain your answer.
-
In a local factory, 20 % of the assembly line workers make $5 per hour and 80 % earn $8 per hour. The union computes the mean hourly wage by randomly drawing five workers. Write the sampling...
-
Do the states play a role in merger regulation, or is it all done at the federal level?
-
The Tate Company began 2010 with a Retained Earnings account balance of $180,000. During 2010, the following eight events occurred and were properly recorded by the company: 1. Bonds payable with a...
-
Portal Manufacturing has total fixed costs of $520,000. A unit of product sells for $15 and variable costs per unit are $11. a). Prepare a contribution margin income statement showing predicted net...
-
The principal strains at a point on the aluminum surface of a tank are 1 = 630(10 -6 ) and 2 = 350(10 -6 ). If this is a case of plane stress, determine the associated principal stresses at the...
-
A uniform edge load of 500 lb/in. and 350 lb/in. is applied to the polystyrene specimen. If the specimen is originally square and has dimensions of a = 2 in., b = 2 in., and a thickness of t = 0.25...
-
A material is subjected to principal stresses Ï x and Ï y . Determine the orientation u of the strain gage so that its reading of normal strain responds only to Ï y and not Ï x ....
-
The following is a list of items that could be included in the intangible assets section of the balance sheet. (a) Indicate which items on the list below would generally be reported as intangible...
-
How does organizational culture intersect with broader societal norms, values, and trends, and what challenges and opportunities does this present for fostering inclusivity, diversity, and ethical...
-
Textile Crafts Company (TCC) sells craft kits and supplies to retail outlets and through online sites such as Etsy.com. Some of the items are manufactured by TCC, while others are purchased for...

Study smarter with the SolutionInn App


