The wave functions for the 1s and 2s orbitals are as follows: where a 0 is a
Question:
The wave functions for the 1s and 2s orbitals are as follows: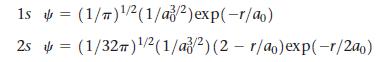
where a0 is a constant (a0 = 53 pm) and r is the distance from the nucleus. Use a spreadsheet to make a plot of each of these wave functions for values of r ranging from 0 pm to 200 pm.
Describe the differences in the plots and identify the node in the 2s wave function.
Transcribed Image Text:
1s (1/)¹/2 (1/a/2) exp(-r/ao) 2s (1/32π) ¹/2(1/a/2) (2 r/ao)exp(-r/2a0) = -
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
The plot for the 2s wave function extends below the xaxis The ...View the full answer

Answered By

Amit Choudhary
I'm new in this profession regarding online teaching but previously i used to teach students near my college. I am teaching on online platform since last year and got good support from the students. I'm teaching on platforms like chegg and vedantu and also at my home in free time.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
We have seen that the wave functions of hydrogen-like atoms contain the nuclear charge Z for hydrogen-like atoms and ions, but modified through equation (9.3) to account for the phenomenon of...
-
The wave functions for a particle in a box (see Fig. 40.12a) are zero at certain points. Does this mean that the particle cant move past one of these points? Explain. Fig.40.12a () ) %3D 3 3D 2 31 ...
-
(a) With reference to Figure 6.19, what is the relationship between the number of nodes in an s orbital and the value of the principal quantum number? Figure 6.19 (b) Identify the number of nodes;...
-
Using the SEDAR database, find the most recent annual reports for two Canadian retailers (e.g., Loblaw, Rona, Danier Leather). Required: a. Based on the information provided in the companies audited...
-
Garcia Corporation experienced a fire on December 31, 2010, in which its financial records were partially destroyed. It has been able to salvage some of the records and has ascertained the following...
-
An L-C circuit containing an 80.0-mH inductor and a 1.25-nF capacitor oscillates with a maximum current of 0.750 A. Calculate: (a) The maximum charge on the capacitor and (b) The oscillation...
-
In 2014, Barker contacted Price about a van Price had advertised for sale. The advertisement described the van as a 1994 Ford E350. Barker and Price agreed to meet, and, on April 9, Barker inspected...
-
The following sample information was obtained by taking four doughnuts per hour for twelve hours from Fawcett Bakerys doughnut process and weighing them: For the data shown above a. Find the x and R...
-
I hired a carpenter and an apprentice to do some work at my business. They worked two days. On the first day I was billed $210 for 11 hours of work by the main carpenter and for 7 hours of work by...
-
Before quantum mechanics was developed, Johannes Rydberg developed an equation that predicted the wavelengths (l) in the atomic spectrum of hydrogen: In this equation, R is a constant and m and n are...
-
The energy of a vibrating molecule is quantized much like the energy of an electron in the hydrogen atom. The energy levels of a vibrating molecule are given by the equation: where n is a quantum...
-
In Exercises find an equation of the tangent line to the graph of the function at the given point. (x) = e x-4 , (4,1)
-
Invest, minimum Ghe 500 in any investment instrument of your choice from any licensed financial institution You are required to 1. Identify your investment horizon 2. State the return expected from...
-
Determine the electric field and potential at a point horizontally -2m and vertically 6 m from a 40 nC charge. g X + Electric field = Eat x=-2, y=6 from q Potential= V at x=-2, y=6 from q = 11.25...
-
Consider the algorithm we discussed in class for finding strongly connected components of a directed graph G = (V,E) by using BFS. Bob wants to decompose G into its strongly connected components....
-
Write a report using the following format. i. Introduction - introduce the background of the case study "Beyond Meat: Changing Consumer's Meat Preference". Suggestion of 200 words. ii. Content 1.0 -...
-
Lisa H., a registered nurse, has worked triage in the Emergency Department in a regional midwestern hospital for 12 years. Originally from New Jersey, she was a nonpracticing Jew until her late 20s....
-
Determine the missing amounts (in millions) for the condensed balance sheets shownbelow. Assets Liabilities Stockholders' equity Costco Target Wal-Mart S23,815 $44,533 $ (C) 12,986 (b) 97,777 15,347...
-
For a Poisson process of rate , the Bernoulli arrival approximation assumes that in any very small interval of length , there is either 0 arrivals with probability 1- or 1 arrival with probability ....
-
Does the average length of a quantum harmonic oscillator depend on its energy? Answer this question by referring to the harmonic potential function shown in Figure 18.7. The average length is the...
-
Does the bond length of a real molecule depend on its energy? Answer this question by referring to Figure 18.7. The bond length is the midpoint of the horizontal line connecting the two parts of V...
-
Why can the angular momentum vector lie on the z axis for two-dimensional rotation in the xy plane but not for rotation in three-dimensional space?
-
Why do older people report feeling younger than they really are? What kind of older people were you familiar with as a child? Did these experiences fashion your stereotypes of the elderly? If not,...
-
How would you describe your self-image? Which aspects of your self-concept would you like to change? Do you basically like yourself? Are you more self-confident in some situations than in others? Are...
-
Aids in making decisions. Reread the section on aids (such as the balance sheet method) to making sounder decisions. Then select one of the poorer decisions youve made and review this decision in...

Study smarter with the SolutionInn App


