We have seen that the wave functions of hydrogen-like atoms contain the nuclear charge Z for hydrogen-like
Question:
We have seen that the wave functions of hydrogen-like atoms contain the nuclear charge Z for hydrogen-like atoms and ions, but modified through equation (9.3) to account for the phenomenon of shielding or screening. In 1930, John C. Slater devised the following set of empirical rules to calculate a shielding constant for a designated electron in the orbital ns or np:
(i) Write the electron configuration of the element, and group the subshells as follows: (1s), (2s, 2p), (3s, 3p), (3d), (4s, 4p), (4d), (4f), (5s, 5p), etc.
(ii) Electrons in groups to the right of the (ns, np) group contribute nothing to the shielding constant for the designated electron.
(iii) All the other electrons in the (ns, np) group shield the designated electron to the extent of 0.35 each.
(iv) All electrons in the n - 1 shell shield to the extent of 0.85 each.
(v) All electrons in the n - 2 shell, or lower, shield completely—their contributions to the shielding constant are 1.00 each.
When the designated electron being shielded is in an nd or nf group, rules (ii) and (iii) remain the same but rules (iv) and (v) are replaced by
(vi) Each electron in a group lying to the left of the nd or nf group contributes 1.00 to the shielding constant.
These rules are a simplified generalization based on the average behavior of different types of electrons.
Use these rules to do the following:
(a) Calculate Zeff for a valence electron of oxygen.
(b) Calculate Zeff for the electron in Cu.
(c) Calculate Zeff for a electron in Cu.
(d) Evaluate the Zeff for the valence electrons in the group 1 elements (including H), and show that the ionization energies observed for this group are accounted for by using the Slater rules.
(e) Evaluate Zeff for a valence electron in the elements Li through Ne, and use the results to explain the observed trend in first ionization energies for these elements.
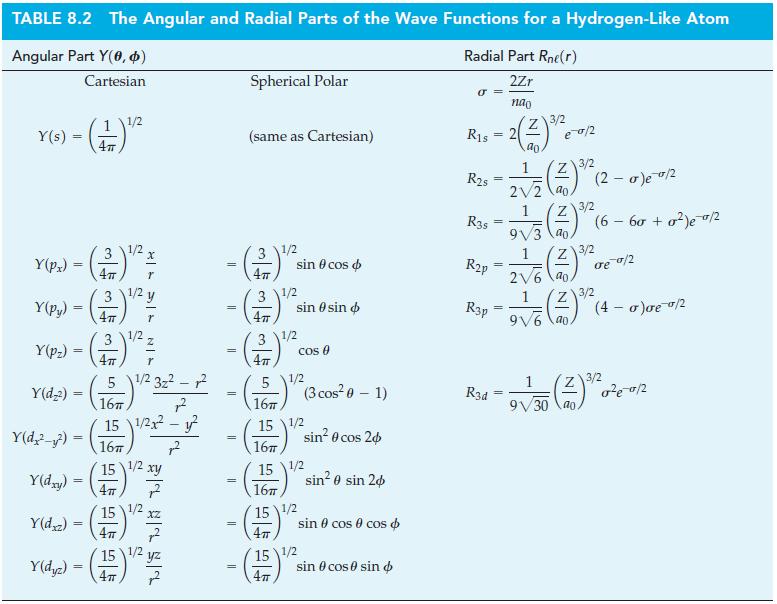
(f) Using the radial functions given in Table 8.2 and Zeff estimated with the Slater rules, compare plots of the radial probability for the 3s, 3p, and 3d orbitals for the H atom and the Na atom. What do you observe from these plots regarding the effect of shielding on radial probability distributions?
Eq. 9.3
![]()
Table 8.2
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





