Upon combustion, a 0.8233-g sample of a compound containing only carbon, hydrogen, and oxygen produces 2.445 g
Question:
Upon combustion, a 0.8233-g sample of a compound containing only carbon, hydrogen, and oxygen produces 2.445 g CO2 and 0.6003 g H2O. Find the empirical formula of the compound.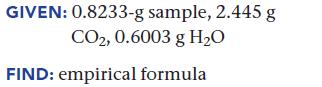
Transcribed Image Text:
GIVEN: 0.8233-g sample, 2.445 g CO2, 0.6003 g H₂O FIND: empirical formula
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
2445 g CO X 005556 1 mol CO 4401 g CO mol CO 06003 gHO ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A compound containing only carbon and hydrogen produces 113.49 g CO2 and 54.36 g H2O when combusted. What is the empirical formula of this compound? If it has a molar mass of 86.20 g/mol, what is its...
-
Write a program "three.c" which contains a function (named "three") that takes two arguments: a pointer to an int and a pointer to a char. The function should return a float. This function should...
-
Upon combustion, a compound containing only carbon and hydrogen produces 1.83 g CO 2 and 0.901 g H 2 O. Find the empirical formula of the compound. GIVEN: 1.83 g CO, 0.901 g HO FIND: empirical formula
-
Selling price Variable costs: Direct materials Direct labour Variable manufacturing overhead Total variable cost Contribution margin Contribution margin ratio Contribution margin per labour hour O...
-
Kaleb Konstruction, Inc., has the following mutually exclusive projects available. The company has historically used a three-year cutoff for projects. The required return is 10 percent. (a) Calculate...
-
Willow Enterprises is considering the acquisition of Steadfast Corp. in a stock swap transaction. Currently, Willows stock is selling for $45 per share. Although Steadfasts shares are currently...
-
Liberty's inventory turnover during 2007 was a. 6 times b. 7 times c. 8 times d. Not determinable from the data given
-
The trial balance of Neal Company as of January 31, 2019, after the company completed the first month of operations, is shown in the partial worksheet below. INSTRUCTIONS 1. Record the trial balance...
-
In order to ensure the competitiveness of operation management, the quality assurance must always be part of organizations' management priorities. Briefly explain the concept and features of Quality...
-
Box and Liu (1999) describe an experiment flying paper helicopters where the objective is to maximize flight time. They used the central composite design shown in Table 14E.9. Each run involved a...
-
What are functionalized hydrocarbons? Cite an example of a functionalized hydrocarbon.
-
What is the difference between an alkane, an alkene, and an alkyne?
-
Consider the avocado data within the Case Study. What additional data fields might be necessary to explore the following questions: a. What is the average income of customers in the cities that yield...
-
What accounting control principles are applicable to the recording of cash receipts and sales adjustments transactions?
-
Identify the control procedures that offer reasonable assurance that cash receipts are deposited intact daily.
-
Briefly discuss the use of cash registers, prelists, and daily cash summaries in controlling cash receipts.
-
Describe the activities of each department that participates in the execution of cash receipts transactions.
-
How do you graphically depict the OE architecture that includes detail interactions of the Higher-Order Systems and Physical Environment domains?
-
While searching through your class notes, you stumble across the following forecasting model: Demand = (35,000 + 4.8 * period) seasonal index SEASONAL INDICES Summer ............ 1.25...
-
a) Calculate the goodwill that was paid by Major Ltd on the acquisition of Minor Ltd. [10 marks] b) Prepare the consolidated statement of financial position for Major Ltd at 31 July 20X8. [30 marks]...
-
What is the physical basis for the experimental result that U is a function of V at constant T for a real gas? Under what conditions will U decrease as V increases?
-
Why didnt Joule change his experiment to make C surroundings /C system approx = 0.001 to increase the sensitivity of the apparatus?
-
Why does the relation C P > C V always hold for a gas? Can C P < C V be valid for a liquid?
-
PCU Corporation's checkbook balance on December 31, 2015 was P160,000. On the same date, PCU held the following items in its safe: A P50,000 check payable to PCU, dated January 2, 2016, was not...
-
A stock has an expected return of 15percent, its beta is 1.50, and the expected return on the market is12 percent. What must the risk-free rate be? (Roundyour answer to 2 decimal places. (e.g.,...
-
Summarize the NY Times article. Do you agree or disagree with the article? How do you feel about inflation? How have you been impacted by inflation?...

Study smarter with the SolutionInn App


