Use standard enthalpies of formation to calculate H rxn for each reaction. 2 HO(l) +2 SO(8) SO3(8)
Question:
Use standard enthalpies of formation to calculate ΔH°rxn for each reaction.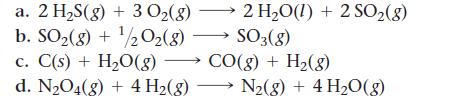
Transcribed Image Text:
2 H₂O(l) +2 SO₂(8) SO3(8) a. 2 H₂S(g) + 3 O₂(g) b. SO2(g) + ¹/2O2(8) c. C(s) + H₂O(g) →→→ CO(g) + H₂(g) d. N₂O4(g) + 4 H₂(g) N₂(g) + 4H₂O(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To calculate Hrxn using standard enthalpies of formation ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculation of H rxn for Part I 0 1. Assume the heat capacity of the final solution is 4.184 J K-1 g-1. Using the final mass of the solution in the calorimeter, calculate q contents from equation...
-
Suppose that you sell a product that is demanded by two different segments of the population (call them X and Y). Suppose that the inverse demand from segment X is Px = 200-2Qx and the inverse demand...
-
An important step in the production of sulfuric acid is the oxidation of SO 2 to SO 3 . Formation of SO 3 from the air pollutant SO 2 is also a key step in the formation of acid rain. (a) Use...
-
The following are the Year 9 income statements of Kent Corp. and Laurier Ltd. Additional Information ¢ Kent acquired its 40% interest in the common shares of Laurier in Year 3 at a cost of...
-
A new accountant at Nicholsen Inc. is trying to identify which of the amounts shown on page 368 should be reported as the current asset Cash and cash equivalents in the year-end balance sheet, as of...
-
(c) What are the largest and smallest values of the spin angular momentum (in terms of h) for the electron in part (a)? (d) What are the largest and smallest values of the orbital angular momentum...
-
Continuing to focus on evidence associated with the act, concealment, and conversion, use the evidentiary material to continue the examination. In addition, as the examiner also start to think of...
-
Erickson Company sponsors a defined benefit pension plan. The corporation??s actuary provides the following information about the plan. Instructions(a) Compute the actual return on the plan assets in...
-
Discuss why people often resist new ideas such as the Relational Model in Business Logic ?
-
Top fuel dragsters and funny cars burn nitromethane as fuel according to the balanced combustion equation: Calculate the standard enthalpy of formation (H f ) for nitromethane. 2 CH3NO(1) + /2O(g) 2...
-
Write an equation for the formation of each compound from its elements in their standard states, and find H rxn for each. a. NO 2 (g) b. MgCO 3 (s) c. C 2 H 4 (g) d. CH 3 OH(l)
-
Montana Matt's Golf Inc. was formed on July 1, 2013, when Matt Magilke purchased the Old Master Golf Company. Old Master provides video golf instruction at kiosks in shopping malls. Magilke plans to...
-
what is lowe's market segmentation ?
-
Why should it study the link between health/biology and psychology?
-
Please evaluate the impact of Gestalt Psychology to the American Psychology scene.?
-
'In psychology, studying personal experience should be more important than measuring traits and abilities.' Critically evaluate this statement, drawing on evidence from social psychology and the...
-
William James is the father of American Psychology. His Functionalist view was different than the psychology that was emerging in Europe at the same time. Discuss functionalism and describe how...
-
The cash register tape for Bluestem Industries reported sales of $6,871.50. Record the journal entry that would be necessary for each of the following situations. (a) Cash to be accounted for exceeds...
-
Find the cross product a x b and verify that it is orthogonal to both a and b. a = (t, 1, 1/t), b = (t 2 , t 2 , 1)
-
Why is the multiplet splitting for coupled spins independent of the static magnetic field?
-
Why does the H atom on the OH group not lead to a multiplet splitting of the methyl hydrogens of ethanol?
-
Why are the multiplet splittings in Figure 28.9 not dependent on the static magnetic field? J12 J42 (yB/2m)(01- 2) Frequency Intensity -----
-
Jasdeep Prudu started a new incorporated business during the current year. The corporation, called Prudu Ltd . , will have a fiscal period of July 1 to June 3 0 and was incorporated on April 1 5 of...
-
Ashely should focus on promoting her software through personal selling because she is offering a B2B solution. She designed 4 versions of her software to meet the needs of different customers, with...
-
How has technology allowed banks to expand their marketing channels beyond branches? Could you describe four (4) technology-related distribution channels for banking services. Also please analyze...

Study smarter with the SolutionInn App


