Use the BornHaber cycle and data from Appendix IIB, Chapter 9, and this chapter to calculate the
Question:
Use the Born–Haber cycle and data from Appendix IIB, Chapter 9, and this chapter to calculate the lattice energy of KCl. (ΔHsub for potassium is 89.0 kJ/mol.)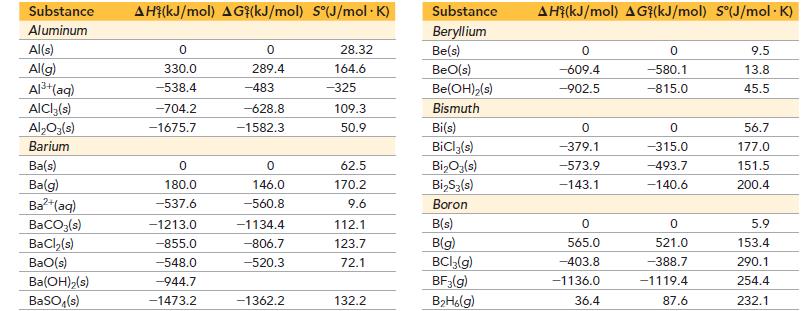
Transcribed Image Text:
Substance Aluminum Al(s) Al(g) Al³+(aq) AlCl3(s) Al₂O3(s) Barium Ba(s) Ba(g) Ba²+(aq) BaCO3(s) BaCl₂(s) BaO(s) Ba(OH)₂(s) BaSO4(s) AH (KJ/mol) AG (kJ/mol) S°(J/mol.K) 0 330.0 -538.4 -704.2 -1675.7 0 180.0 -537.6 -1213.0 -855.0 -548.0 -944.7 -1473.2 0 289.4 -483 -628.8 -1582.3 0 146.0 -560.8 -1134.4 -806.7 -520.3 -1362.2 28.32 164.6 -325 109.3 50.9 62.5 170.2 9.6 112.1 123.7 72.1 132.2 Substance Beryllium Be(s) BeO(s) Be(OH)₂ (s) Bismuth Bi(s) BiCl3(s) Bi₂O3(s) Bi₂S3(s) Boron B(s) B(g) BCl3(g) BF3(g) B₂H6(g) AH (kJ/mol) AG (kJ/mol) S°(J/mol. K) 0 -609.4 -902.5 0 -379.1 -573.9 -143.1 0 565.0 -403.8 -1136.0 36.4 0 -580.1 -815.0 0 -315.0 -493.7 -140.6 0 521.0 -388.7 -1119.4 87.6 9.5 13.8 45.5 56.7 177.0 151.5 200.4 5.9 153.4 290.1 254.4 232.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
From the lattice energy of KCl in Table 9.1 and the ionization energy of K and electron affinity of Cl in Tables 8.2 and 8.3, calculate the DH° for the reaction K(g) + Cl(g) KCl(s)
-
Calculate the lattice energy of CaCl 2 using a Born- Haber cycle and data from Appendices F and L and Table 7.5. Data given in Table 7.5 TABLE 7.5 2nd Period 1st 2nd 3rd 3rd Period 1st 2nd 3rd 4th...
-
The value of Go for the reaction 2C4H10(g) + 13O2(g) 8CO2(g) + 10H2O(l) is 5490. kJ. Use this value and data from Appendix 4 to calculate the standard free energy of formation for C4H10(g).
-
+9.33 x 10 C +91 Find the net force on 92. 0.180 m +4.22 x 10 C +92 0.230 m- -8.42 x 10 C 93 F = force exerted on q2 by 91 F3 = force exerted on 92 by 93 F = 10.9 N F3 = 6.04 N Remember: Forces...
-
An internal auditor should have a sound understanding of basic data processing concepts such as data organization and storage in order to adequately evaluate systems and make use of retrieval...
-
A 2-kg block is released 4 m from a massless spring with a force constant k = 100 N/m that is fixed along a frictionless plane inclined at 30, as shown in Figure. (a) Find the maximum compression of...
-
Defendant Monty J. Person began working for Garage Solutions, LLC, in March 2015. Three months into his employment, Person was sent by the owner of Garage Solutions, Mark Fontenot, to Rexburg, Idaho,...
-
The Tall Oaks Wood Products Company is considering purchasing timberland for $5 million that would provide a future source of timber supply for the companys operations over the next 10 years....
-
Draw a diagram with the wage-setting relation and price-setting relation. Label your wage-setting curve WS and your price setting line PS. Label the y axis the Real Wage and the x axis the...
-
Write the electron configuration for N. Then write the Lewis symbol for N and show which electrons from the electron configuration are included in the Lewis symbol.
-
How does the electron sea model explain the conductivity of metals? The malleability and ductility of metals?
-
Explain, in broad terms, the strategic options available to marketers regarding advertising on the Internet. Discuss in particular the difference between banner advertising and pop-ups and how these...
-
In their study, Vardeman and Ray found that the number of accidents per hour at an industrial plant is exponentially distributed with a mean = .5. Use the formula f(t) = e - t to determine each of...
-
At an antiques auction, the winning bids were found to be uniformly distributed between $500 and $2,500. What is the probability that a winning bid was less than $1,000? What is the probability that...
-
For each of the following samples drawn from normal populations, find the best estimates for , 1 , , and the standard deviation of X. (a) 4, 10, 2, 8, 4, 14, 12, 8, 30 (b) 4, 2, 6, 0, 6, 2, 4, 0, 4...
-
In the landmark Dodge v. Ford case in 1919, the Michigan State Supreme Court determined whether Henry Ford could withhold dividends from the Dodge brothers (and other shareholders of Ford Motor...
-
Suppose you construct a 95 % confidence interval for the mean of an infinite population. Will the interval always be narrower when is known than when is unknown?
-
For what reasons may the NAIRU increase?
-
Provide an example of an aggressive accounting practice. Why is this practice aggressive?
-
For the water tank shown in Fig. 4.43, compute the magnitude and location of the total force on the inclined wall. 8 ft Water 60 15 ft 10 ft
-
For the orange-drink tank shown in Fig. 4.32, compute the magnitude and location of the total force on each vertical end wall. The tank is 3.0 m long. 30 Orange drink (sg - 1.10) 3.0 m 4.6 m 2.4-m...
-
For the orange-drink tank shown in Fig. 4.32, compute the magnitude and location of the total force on the vertical back wall. The tank is 3.0 m long. 30 Orange drink (sg - 1.10) 3.0 m 4.6 m 2.4-m...
-
Not all convention planners need to reach out to the media, but those that do should be sure to have carefully built relationships and manage their messaging. In this discussion, news release for...
-
In the Advertising Industry, there are different forms of added value that could be considered for each media. Choose one media and describe one of the added value options that could be considered...
-
1. Tobi Vail is a ski instructor and has an annual gross pay of $19,250. His exemptions total $1500. The state tax on the first $2500 is 1.5% and amounts over $2500 is 3%. What is the state income...

Study smarter with the SolutionInn App


