Use the dipole moments of HF and HCl (given at the end of the problem) together with
Question:
Use the dipole moments of HF and HCl (given at the end of the problem) together with the percent ionic character of each bond (Figure 10.10) to estimate the bond length in each molecule.
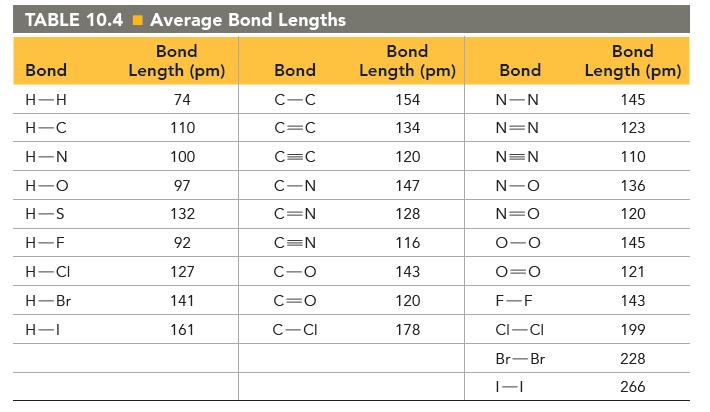
How well does your estimated bond length agree with the bond length in Table 10.4?![]()
Transcribed Image Text:
HCl p = 1.08 D HF м = 1.82 D
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
These values ...View the full answer

Answered By

Simon kingori
I am a tier-one market researcher and content developer who has been in this field for the last six years. I’ve run the freelancing gamut; from market research, data mining and SEO/SMM to copywriting, Content Development, you name it, I’ve done it. I’m extremely motivated, organized and disciplined – you have to be to work from home. My experience in Freelancing is invaluable- but what makes me a cut above the rest is my passion to deliver quality results to all my clients- it’s important to note, I've never had a dissatisfied client. Backed by a Masters degree in Computer Science from MOI university, I have the required skill set and burning passion and desire to deliver the best results for my clients. This is the reason why I am a cut above the rest. Having taken a Bsc. in computer science and statistics, I deal with all round fields in the IT category. It is a field i enjoy working in as it is dynamic and new things present themselves every day for research and exploration.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
(a) Use Figure 3.18 to rank the quantities f'(1), f'(2), f'(3) from smallest to largest. (b) Confirm your answer by calculating the quantities using the formula, f(x) = 2e x 3x 2 x. 8 4 -4 -8 1 3...
-
The O-H bond lengths in the water molecule (H2O) are 0.96 Ã, and the H-O-H angle is 104.5º. The dipole moment of the water molecule is 1.85 D. (a) In what directions do the bond dipoles...
-
The dipole moments of the hydrogen halides decrease from HF to HI (see Table 10.3). Explain this trend?
-
Either: Prove that k-means will produce k clusters, allnonempty, OR: Give anexample of a set D of data points (with no repeated data point), avalue for k(k
-
Lusive Corporation has a standard cost system in which it applies overhead to products based on the standard direct labor-hours allowed for the actual output of the period. Data concerning the most...
-
Consider an LTI system that is stable and for which H(z), the z-transform of the impulse response, is given by Suppose x[n], the input to the system, is a unit step sequence. (a)Find the output y[n]...
-
Plaintiff grounds manager sued a manufacturer, Monsanto, alleging that herbicide use caused his non-Hodgkins lymphoma. The jury awarded the plaintiff \($39.3\) million in compensatory damages and...
-
Roy Creasey Enterprises, a machine shop, is planning to move to a new, larger location. The new building will be 60 feet long by 40 feet wide. Creasey envisions the building as having six distinct...
-
a. Write a story problem for the expression 6 x b. Find the value of the expression. Show or explain your work. da f
-
A 0.167-g sample of an unknown acid requires 27.8 mL of 0.100 M NaOH to titrate to the equivalence point. Elemental analysis of the acid gives the following percentages by mass: 40.00% C, 6.71% H,...
-
The main component of acid rain (H 2 SO 4 ) forms from the SO 2 pollutant in the atmosphere via these steps: Draw the Lewis structure for each of the species in these steps and use bond energies and...
-
Using each digit not more than once, how many even four-digit numbers can be made from the digits 1, 2, 3, 4, 5, 6 and 7?
-
Donald is resident and domiciled in the UK. He is not a Scottish taxpayer. He has the following income in tax year 2020-21: Donald claims only the personal allowance. Compute the amount of income tax...
-
Quiver Ltd is VAT-registered. During the quarter to 31 March 2021, the company made the following supplies of goods and services: The input tax which is attributed to taxable supplies includes 1,710...
-
Q Ltd prepares accounts to 31 March each year. The company made the following two disposals of chargeable assets during the year to 31 March 2021: (1) 1,250 shares in Hentic Ltd were sold on 28 June...
-
For many years, W Ltd has owned 70% of the issued shares of X Ltd, 30% of the issued shares of Y Ltd and 80% of the issued shares of Z Ltd. All four companies are UK resident and all of the shares of...
-
Neil has been in business for many years preparing accounts to 5 April each year. His profits in a typical year (adjusted for tax purposes) are approximately 50,000 and his drawings are 3,000 per...
-
The following are the December 31, 2009 post-closing trial balance and the December 31, 2010 adjusted trial balance of the Adair Company: A review of the accounting records reveals the following...
-
Identify Thank You mission, strategy and core competencies. Identify strategy changes that have taken place at Thank You since its founding in 2008. Your answer must in text references and must be...
-
The bar has a thickness of 1 in. and the allowable bending stress is Ï allow = 30 ksi. Determine the maximum moment M that can be applied. 0.5 in. 6 in. 4 in.
-
The bar has a thickness of 1 in. and is subjected to a moment of 3 kip · ft. Determine the maximum bending stress in the bar. 0.5 in. 6 in. 4 in.
-
The bar has a thickness of 0.5 in. and the allowable bending stress is Ï allow = 20 ksi. Determine the maximum moment M that can be applied. 0.30 in. 6 in. 2 in.
-
You are a regional manager at the Walk-In Closet clothing store and you just received the most recent feedback from a mystery shopper's in-store experience report. One thing that caught your eye in...
-
What is the health care manager's role in creating a culturally responsible organization?
-
Explain the importance of ethical expectations and modeling ethical practices in le within education. your future

Study smarter with the SolutionInn App


