What volume (in mL) of a soft drink that is 10.5% sucrose (C 12 H 22 O
Question:
What volume (in mL) of a soft drink that is 10.5% sucrose (C12H22O11) by mass contains 78.5 g of sucrose?
(The density of the solution is 1.04 g/mL.)
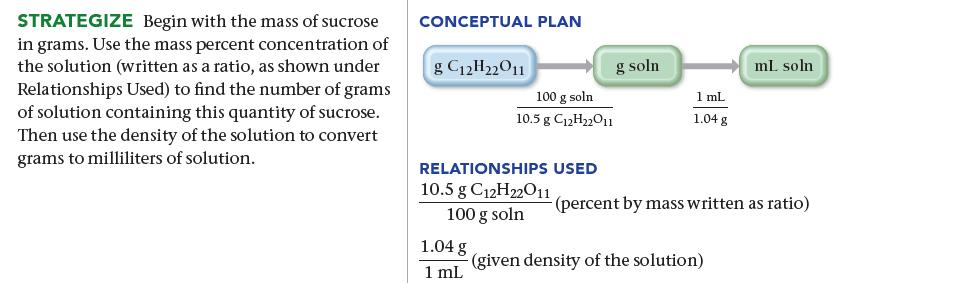
Transcribed Image Text:
SORT You are given a mass of sucrose and the concentration and density of a sucrose solution, and you are asked to find the volume of solution containing the given mass. GIVEN: 78.5 g C12H22O11 10.5% C12H22011 by mass density = 1.04 g/mL FIND: ML
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
785 g C12H220...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Experiment 2: Osmosis - Direction and ConcentrationGradients In this experiment, we will investigate the effect of soluteconcentration on osmosis. A semi-permeable membrane (dialysistubing) and...
-
The amount of a soft drink that goes into a typical 12-ounce can varies from can to can. It is normally distributed with an adjustable mean and a fixed standard deviation of 0.05 ounce. a. If...
-
Calculate the vapor pressure at 25 C of a solution containing 99.5 g sucrose (C 12 H 22 O 11 ) and 300.0 mL water. The vapor pressure of pure water at 25 C is 23.8 torr. Assume the density of water...
-
Briefly explain the DHCP lease process. What packets are sent and when are they sent? (5 marks) Question 2 any three advantages of IPv6 over IPv4? How many classes are there in IPv4 and what is the...
-
What is customer value? How is customer value related to a cost leadership strategy? To a differentiation strategy? To strategic positioning?
-
The force exerted by the earth on a particle of mass m a distance r from the center of the earth has the magnitude GM E m/r 2 = mgR E 2 /r 2 . (a) Calculate the work you must do against gravity to...
-
How would the primordial helium content of the universe have been affected if the present cosmic background radiation temperature was \(27 \mathrm{~K}\) instead of \(2.7 \mathrm{~K}\) ? What about...
-
Division A manufactures picture tubes for TVs. The tubes can be sold either to Division B of the same company or to outside customers. Last year, the following activity was recorded in Division A:...
-
Give the steps required to use the Oracle Enterprise Manager to monitor the performance of the Oracle Database and identify any potential performance bottlenecks.
-
What does it mean to say that a substance is soluble in another substance? Which units are used in reporting solubility?
-
A 500.0-mL sample of pure water is allowed to come to equilibrium with pure oxygen gas at a pressure of 755 mmHg. What mass of oxygen gas dissolves in the water? (The Henrys law constant for oxygen...
-
A restaurant achieved $150,000 in net income. The restaurants balance sheet shows assets of $600,000, and liabilities of $200,000. What is this restaurants return on equity ratio? a. 37.5% b. 0.375%...
-
Tammy Faye Jones owned three passive activities in 2019 (she had no suspended losses prior to 2019): How much is her passive loss deduction (against nonpassive income) and suspended loss for each...
-
Charles Adams, who is single, retired on March 3, 2019, at the age of 65. For the year 2019, he receives the following income: What is Charles' modified AGI? What amount, if any, of his Social...
-
Return to the bicycle manufacturer NatBike in Exercise 6. Assume that the plant has a capacity of 20,000 bicycles. If additional capacity can be added at a cost of $25 per bicycle, how should NatBike...
-
A married couple with two children has earned income of $30,000 with no withholding. Assume they have a taxable income of $100, they paid $2,295 in FICA taxes, and they will receive an earned income...
-
A married couple with two children has $16,000 of earned income. What is the amount of their child and family tax credit?
-
LOreal reports the following for a recent year for the major divisions in its Cosmetics branch. 1. Compute profit margin for each division. State your answers as percents, rounded to two decimal...
-
(a) Use integration by parts to show that (b) If f and g are inverse functions and f' is continuous, prove that (c) In the case where f and t are positive functions and b > a > 0, draw a diagram to...
-
A standard 6-in Schedule 40 steel pipe is carrying 95 gal/min of water. The pipe then branches into two standard 3-in pipes. If the flow divides evenly between the branches, calculate the velocity of...
-
Repeat Problem 6.51 for a 4-in Schedule 80 pipe. Repeat Problem Compute the resulting velocity of flow if 400 gal/min of fluid flows through a 4-in Schedule 40 pipe.
-
Compute the resulting velocity of flow if 400 gal/min of fluid flows through a 4-in Schedule 40 pipe.
-
Develop an argument for the implementation of an HRIS using a risk reduction strategy and an organizational enhancement strategy.
-
You are a senior member of the human resource management (HRM) function in your case study organization (from the previous Assignment: Talent Sourcing Project). You have been asked...
-
How has the global environment changed for U.S. (Hollywood) movie studios? Explain. Feel free to cite examples or describe the evolution in detail. Is this strategic?

Study smarter with the SolutionInn App


