When ammonium chloride (NH 4 Cl) is dissolved in water, the solution becomes colder. a. Is the
Question:
When ammonium chloride (NH4Cl) is dissolved in water, the solution becomes colder.
a. Is the dissolution of ammonium chloride endothermic or exothermic?
b. What can you conclude about the relative magnitudes of the lattice energy of ammonium chloride and its heat of hydration?
c. Sketch a qualitative energy diagram similar to Figure 14.7 for the dissolution of NH4Cl.
d. Why does the solution form? What drives the process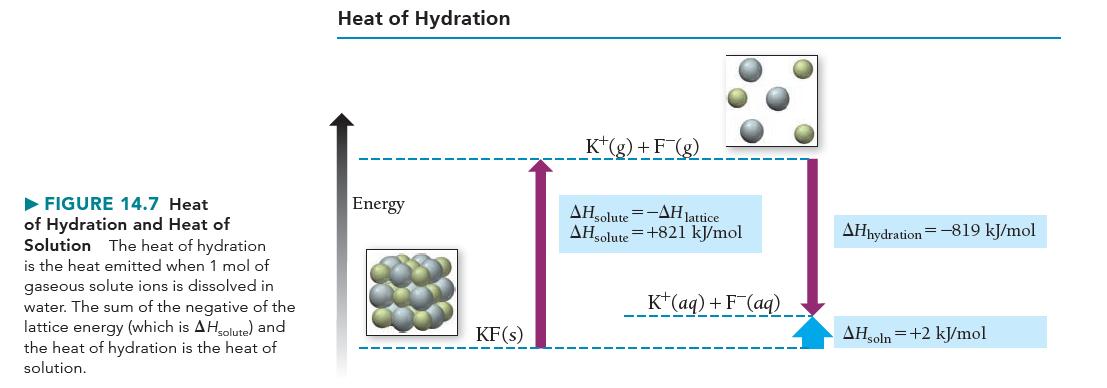
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





