For each compound, would you expect greater solubility in water or in hexane? Indicate the kinds of
Question:
For each compound, would you expect greater solubility in water or in hexane? Indicate the kinds of intermolecular forces that would occur between the solute and the solvent in which the molecule is most soluble.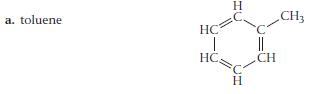
Transcribed Image Text:
a. toluene. HC | HC Н Н || CH CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a Toluene Polarity Nonpolar Expected solubility Greater solubility in hexane Intermolecular forces D...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For each compound, would you expect greater solubility in water or in hexane? Indicate the kinds of intermolecular forces that occur between the solute and the solvent in which the molecule is most...
-
1. Look up and draw the structures for each of the following molecules: (a) glucose (b) naphthalene (c) dimethyl ether (d) alanine For each molecule, would you expect greater solubility in water or...
-
Pick an appropriate solvent from Table 14.3 to dissolve each substance. State the kind of intermolecular forces that would occur between the solute and solvent in each case. a. Isopropyl alcohol...
-
Discuss the impact of commissions in organizations growth?
-
Jerry Goff, president of Harmony Electronics, was concerned about the end-of-the-year marketing report that he had just received. According to Emily Hagood, marketing manager, a price decrease for...
-
From a molecular perspective, briefly explain the mechanism by which clay minerals become hydroplastic when water is added.
-
Walt Disney reports the following information for its two Parks and Resorts divisions. Assume Walt Disney uses a balanced scorecard and sets a target of 85% occupancy in its resorts. Using Exhibit...
-
A tabular analysis of the transactions made during August 2014 by Colaw Company during its first month of operations is shown below. Each increase and decrease in stock- holders equity is explained....
-
Suppose that farmers have a mandatory demand expansion program where all farmers pay a certain amount to fund a promotion program. You have estimated the following market supply and demand functions...
-
When ammonium chloride (NH 4 Cl) is dissolved in water, the solution becomes colder. a. Is the dissolution of ammonium chloride endothermic or exothermic? b. What can you conclude about the relative...
-
Which molecule would you expect to be more soluble in water: CCl 4 or CH 2 Cl 2 ?
-
Add labels to the figure that follows, which illustrates a virus. 0.01 m 2
-
What types of property passing to a spouse are considered terminable interests that would not qualify for the marital deduction?
-
George has a service business and wishes to create an S corporation with a June 30 fiscal year. What problems might George encounter in his fiscal-year selection?
-
What tax year options are available to a partnership?
-
Why is the partner's basis in distributed property limited to his/her basis in the partnership interest prior to the distribution?
-
Rhoda and Mike are both full-time students who married late in the year. Rhoda had $1,800 in wages from a part-time job and Mike had $950 in dividend income. They filed a joint return and received a...
-
Render Co. CPA is preparing activity- based budgets for 2013. The partners expect the firm to generate bill-able hours for the year as follows: Data entry . . . . . . . . . 2,200 hours Auditing . . ....
-
What is beacon marketing? What are digital wallets?
-
Imagine a skydiver who waits a long time before opening her parachute. For simplicity, assume she moves along a straight line. (a) In what direction is the skydiver moving (what is the direction of...
-
In our discussion of the block and tackle in Figure 3.23, we claimed that when the right end of the string is lifted through a distance L, the body of the pulley is lifted a distance L/2. Give a...
-
An astronaut measures her mass and her weight on Earth and again when she reaches the Moon. Which one of these quantities changes as a result of this trip and which one does not? Explain.
-
Use the Trapezoidal rule and Simpson's rule with four (4) subintervals to approximate the integral edx. Perform all calculations and the answer correct to four (4) decimal places. (7 marks)
-
Now is the time to be on the Interneteven if you do not think your target customer is a potential Web surfer. Every business should have a website. How do you increase your chances of successfully...
-
1) Why is it best to mix and match targeting tactics? Why can't just one type of online advertising method take care of everything? 2) Compare and contrast the different types of targeting:...

Study smarter with the SolutionInn App


