Calculate the pH of the following solutions: a. 1.2 M CaBr b. 0.84 M C6H5NH3NO3 (K for
Question:
Calculate the pH of the following solutions:
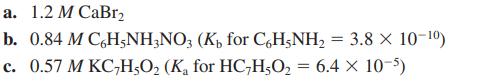
Transcribed Image Text:
a. 1.2 M CaBr₂ b. 0.84 M C6H5NH3NO3 (K₁ for C6H5NH₂ = 3.8 × 10-¹⁰) c. 0.57 M KC₂H5O₂ (K₁ for HC-H₂O₂ = 6.4 x 10-5)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
Solution 1 12 M CaBr CaBr is a salt of a strong base CaOH and a strong acid HBr When dissolved in wa...View the full answer

Answered By

Arun kumar
made more than four thousand assignments
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Calculate the pH of each of the following solutions (Ka and Kb values are given in Appendix D): (a) 0.095 M propionic acid (C2H5COOH), (b) 0.100 M hydrogen chromate ion (HCrO4-, (c) 0.120 M pyridine...
-
First, write the code to create a single DataFrame object in a function called load_ticket_data(). This function should return the full dataframe and take no parameters (you can assume the ticket...
-
Pioneer Bicycle Shop is an authorized Trek dealer. In order to be an authorized Trek dealer, Pioneer and Trek signed an agreement where Trek agreed to sell Pioneer its entire line of bicycles and...
-
What are the types of production systems in aquaculture?
-
On what did the court base its reasoning for its ruling on this issue?
-
Fill in the Blank. The spring constant denotes the _________ necessary to cause a unit elongation.
-
Determine the diameter of a red brass C83400 bar that is \(8 \mathrm{ft}\) long if it is to be used to absorb \(800 \mathrm{ft} \cdot \mathrm{lb}\) of energy in tension from an impact loading. No...
-
The City of Sandwich purchased a swimming pool from a private operator as of April 1, 2012, for $500,000. Of the $500,000, $250,000 was provided by a one-time contribution from the General Fund, and...
-
Discuss sentiment analysis in relation to threat actors and predicting future attacks. What defenses could be used to combat domain generation algorithms DGA? How helpful is a historical analysis?...
-
Conch Republic Electronics is a midsized electronics manufacturer located in Key West, Florida. The company president is Shelley Couts, who inherited the company. When it was founded over 70 years...
-
Students are often surprised to learn that organic acids, such as acetic acid, contain OOH groups. Actually, all oxyacids contain hydroxyl groups. Sulfuric acid, usually written as H 2 SO 4 , has the...
-
Calculate the pH of each of the following solutions. a. 0.12 M KNO 2 b. 0.45 M NaOCl c. 0.40 M NH 4 ClO 4
-
Under flexible exchange rates, what happens if a country experiences a deficit in its balance of payments? How long can a deficit in the balance of payments persist?
-
Provided below is the activities to be completed in order to accomplish a project. Activities 1-2 Duration in weeks Optimistic time Most likely time Pessimistic time 1 1-3 1-4 2-5 3-5 2 132132 4-6...
-
a) Explain what operation analysis is and using example; discuss the stages in an Operations Analysis study. (8 marks) b) Give an economic interpretation of Hawkins Simon Conditions in the three...
-
a) Nyamakima group of companies has a sponsored a weight loss programme for mothers aged 18-40 years who are obese. The programme nutritionist has set up minimum daily requirement for several kind of...
-
a) Describe info-gap decision theory citing its three models of decision making under uncertainty. [6 Marks] b) Kerubo is dealing with uncertain income situation. She expect to make Ksh. 20,000 per...
-
There are several Tax Credits and Deductions that reduce Tax Liability (the amount of tax you owe the government), including the Earned Income Tax Credit meant to assist those with lower earned...
-
The manager of a tire store in Minneapolis has been concerned with the high cost of inventory. The current policy is to stock all the snow tires that are predicted to sell over the entire winter at...
-
Three forces with magnitudes of 70pounds, 40 pounds, and 60 pounds act on an object at angles of 30, 45, and 135, respectively, with the positive x-axis. Find the direction and magnitude of the...
-
Nitriles undergo alkylation at the α position much like ketones undergo alkylation at the α position. The α position of the nitrile is first deprotonated to...
-
Identify the Michael donor and Michael acceptor that could be used to prepare each of the following compounds via a Michael addition. (a) (b) (c) (d) (e) OEt N
-
The conjugate base of diethyl malonate can serve as a nucleophile to attack a wide range of electrophiles. Identify the product that is formed when the conjugate base of diethyl malonate reacts with...
-
Discuss the steps of an action learning program. Which aspect of action learning do you think is most beneficial for learning? Which aspect is most beneficial for transfer of training? Explain why...
-
I agree with the statement from Bititchi because an organization knowing their position in a VUCA world that builds on their culture shows that the company is thriving on all fronts and if the...
-
In the text, the authors provide a summary of advice HRD should take from adult learning theory for the adult learning planning process. Depending on the nature of the programming, it may not be...

Study smarter with the SolutionInn App


