Consider a solution of an unknown salt having the general formula BHCl, where B is one of
Question:
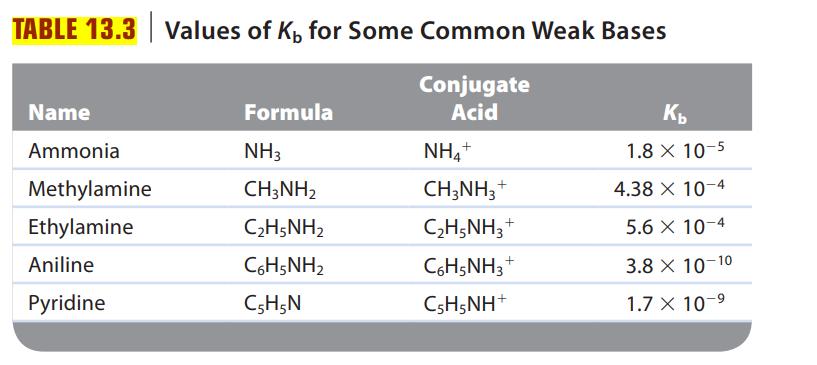
Consider a solution of an unknown salt having the general formula BHCl, where B is one of the weak bases in Table 13.3. A 0.10-M solution of the unknown salt has a pH of 5.82. What is the actual formula of the salt?
Table 13.3.
Transcribed Image Text:
TABLE 13.3 Values of K, for Some Common Weak Bases Conjugate Acid Name Ammonia Methylamine Ethylamine Aniline Pyridine Formula NH3 CH3NH₂ C2H5NH2 CoH5NH2 C5H5N NH4+ CH3NH3 + C₂H5NH3 C6H5NH3 CsH5NH* + + Kb 1.8 X 10-5 10-4 10-4 10-10 10-⁹ 4.38 x 5.6 x 3.8 X 1.7 X
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To determine the actual formula of the unknown salt we need to determine the identity of the weak ba...View the full answer

Answered By

Antony Mutonga
I am a professional educator and writer with exceptional skills in assisting bloggers and other specializations that necessitate a fantastic writer. One of the most significant parts of being the best is that I have provided excellent service to a large number of clients. With my exceptional abilities, I have amassed a large number of references, allowing me to continue working as a respected and admired writer. As a skilled content writer, I am also a reputable IT writer with the necessary talents to turn papers into exceptional results.
4.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
An aqueous solution of an unknown salt of gold is electrolyzed by a current of 2.75 amps for 3.50 hours. The electroplating is carried out with an efficiency of 90.0%, resulting in a deposit of...
-
An aqueous solution of an unknown salt of vanadium is electrolyzed by a current of 2.50 amps for 1.90 hours. The electroplating is carried out with an efficiency of 95.0%, resulting in a deposit of...
-
Separate samples of a solution of an unknown salt are treated with dilute solutions of HBr, H2SO4, and NaOH. A precipitate forms in all three cases. Which of the following cations could the solution...
-
If net assets of a business totalled 200,000 and its total assets on that date amounted to 325,000, its liabilities would amount to: (a) 125,000 (b) 200,000 (c) 525,000 (d) Not sufficient information...
-
Vickie Lynn Smith, an actress and model also known as Anna Nicole Smith, met J. Howard Marshall II in 1991. During their courtship, J. Howard lavished gifts and large sums of money on Anna Nicole,...
-
True or False. An unrestrained system is also known as a semidefinite system.
-
Determine the vertical displacement of end \(B\) of the frame. Consider only bending strain energy. The frame is made using two A-36 steel W460 \(\times 68\) wide-flange sections. 4 m A -3 m- B 20 kN
-
Overall, Steve Edwards, vice president of Marketing at Ditten hoefer's Fine China, is very pleased with the success of his new line of Gem-Surface china plates. Gem-Surface plates are different from...
-
Company XYZ is a wholesale club chain store, such as Costco or Sam's Club. You, as a data analyst, are asked to perform the following data clean up, data transformation, and data calculation tasks in...
-
You have recently been hired by Keafer Manufacturing to work in its newly established treasury department. Keafer Manufacturing is a small company that produces highly customized cardboard boxes in a...
-
Calculate the pH of each of the following solutions. a. 0.12 M KNO 2 b. 0.45 M NaOCl c. 0.40 M NH 4 ClO 4
-
Are solutions of the following salts acidic, basic, or neutral? For those that are not neutral, write balanced equations for the reactions causing the solution to be acidic or basic. The relevant Ka...
-
According to Baer, Wolf, and Risley (1968), what is the difference between basic and applied behavior analysis? (a) basic research is likely to look at any behavior and any variable (b) applied...
-
(a) Explain the meaning of money. (b) Describe in detail three (3) stages recognized by various Writers in the development of money. (c) Discuss the circumstances in which a commercial bank may...
-
(a) Define money as per Crowther (3 marks). (b) "The development of money passed through many stages in accordance with time, place and circumstances with the progress in economic civilization of...
-
a) Explain the meaning of electronic banking and the benefits of e-banking to banking industry (7 marks). b) Discuss four (4) reasons why a bank may dishonor a cheque (8 marks).
-
Critically explain the major types of accounts that are offered to the customers in a banking industry (15 marks).
-
(a) Explain the following negotiable instruments that are negotiable under the law: i) Bill of exchange (2 marks). ii) Bank draft (2 marks). iii) Promissory note (2 marks). b) Describe the methods...
-
Refer to Exercise. Is there evidence of positive first-order autocorrelation?
-
How will relating product contribution margin s to the amount of the constrained resource they consume help a company maximize its profits?
-
Propose an efficient synthesis for the following transformation.
-
For a pair of keto-enol tautomers, explain how IR spectroscopy might be used to identify whether the equilibrium favors the ketone or the enol.
-
Acrolein is an α,β-unsaturated aldehyde that is used in the production of a variety of polymers. Acrolein can be prepared by treating glycerol with an acid catalyst. Propose...
-
I think the Power Distance measure in Hofstede's model (Hofstede Insights, n.d.) is particularly interesting.I led divisions in the U.S., New Zealand, and Thailand.Those three countries represented a...
-
TechEx Repair allows local hardware stores to expand their service offerings to their customers by providing an off-site small engine repair service. Customers bring in small engines such as lawn...
-
Identify and discuss reasons as to why capital structure is either relevant or irrelevant under the conditions of perfect markets. If your boss were to ask you about considering changing your...

Study smarter with the SolutionInn App


