Are solutions of the following salts acidic, basic, or neutral? For those that are not neutral, write
Question:
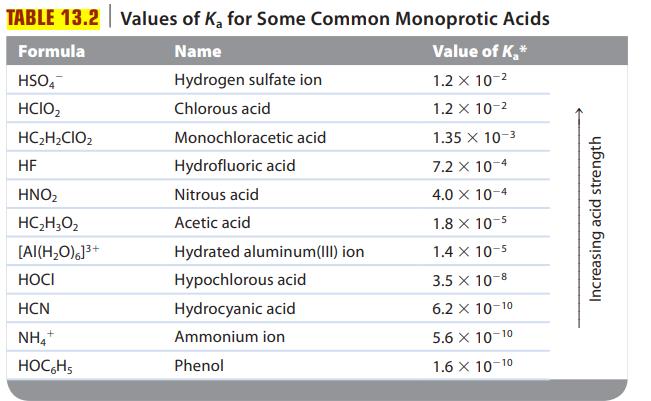
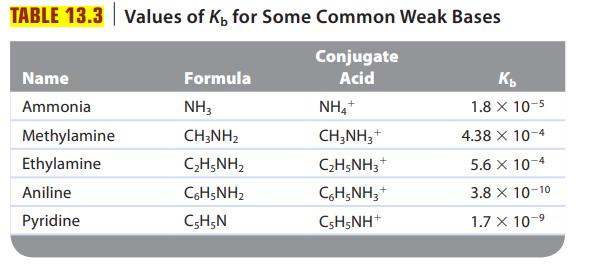
Are solutions of the following salts acidic, basic, or neutral? For those that are not neutral, write balanced equations for the reactions causing the solution to be acidic or basic. The relevant Ka and Kb values are found in Tables 13.2 and 13.3.
Transcribed Image Text:
TABLE 13.2 Values of K₂ for Some Common Monoprotic Acids Name Value of K₂* Hydrogen sulfate ion 1.2 x 10-² Chlorous acid 1.2 x 10-² 1.35 x 10-3 7.2 x 10-4 4.0 X 10-4 Formula HSO4 HCIO₂ HC₂H₂CIO₂ HF HNO₂ HC,H,Oz [AI(H₂O)]³+ HOCI HCN NH4+ HOCHS Monochloracetic acid Hydrofluoric acid Nitrous acid Acetic acid Hydrated aluminum(III) ion Hypochlorous acid Hydrocyanic acid Ammonium ion Phenol 1.8 x 10-5 1.4 x 10-5 3.5 x 10-8 6.2 X 10-10 5.6 X 10-10 1.6 X 10-10 Increasing acid strength
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Solutions of the following salts are acidic basic or neutral Salt Solution Equation NaHS Basic NaHS H2O HS Na H3O NH4Cl Acidic NH4Cl NH4 Cl NaCH3COO B...View the full answer

Answered By

Joemar Canciller
I teach mathematics to students because I love to share what I have in this field.
I also want to see the students to love math and be fearless in this field.
I've been tutoring these past 2 years and I would like to continue what I've been doing.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Lets take a look at the extensive career of Helen Frankenthaler (1928-2011), who found a way to incorporate all of the innovative techniques artists were experimenting with starting in 1945. Her...
-
Will 0.10 M solutions of the following salts be acidic, basic, or neutral? a. Ammonium bicarbonate b. Sodium dihydrogen phosphate c. Sodium hydrogen phosphate d. Ammonium dihydrogen phosphate e....
-
Decide whether solutions of the following salts are acidic, neutral, or basic. a. Ammonium acetate b. Anilinium acetate
-
Which of the following would be the most frequently occurring daily transaction in a retail shop? (a) Paying salary to the sales assistant (b) Sale of goods (c) Payment of rent for the shop premises...
-
James Lillards first wife had a child whom James adopted when he married that childs mother. James fathered other children with her until they divorced in the early 1970s. In 1975, James married his...
-
Calculate the three currents I1, I 2, and I3 indicated in the circuit diagram shown in Fig. 5.00 1 ww 8.00 2 1.00 9:00 . 12:00 L00 10.00 1
-
A university pumps its water from wells located on campus. The falling water table has caused pumping costs to increase, the quantity of water available to decrease, and the quality of water to...
-
Nguyen Company has the following stock outstanding: Common Stock Preferred Stock 60,000 shares ......5,000 shares $1 par value .......$60 par, $3 dividend The amount available for dividends this year...
-
Bleeker Street Pizza and Keste Pizza & Vino are located close to each other on Bleeker Street in the West Village. There are 450 potential customers every day, and suppose that each of them is...
-
Top Quality Appliance-Long Beach has just purchased a franchise from Top Quality Appliance (TQA). TQA is a manufacturer of kitchen appliances. TQA markets its products via retail stores that are...
-
Consider a solution of an unknown salt having the general formula BHCl, where B is one of the weak bases in Table 13.3. A 0.10-M solution of the unknown salt has a pH of 5.82. What is the actual...
-
The K b values for ammonia and methylamine are 1.8 10 -5 and 4.4 10 -4 , respectively. Which is the stronger acid, NH 4 + or CH 3 NH 3 + ?
-
James Kimball, the defendant, was charged with and convicted of attempted unarmed robbery, at a bench trial conducted in early August 1979. He was sentenced to a prison term of from three to five...
-
Mary's Marketplace is comparing her financial statements with her competitor, Mica's Main Grocery. Both Mary and Mica are in their first year of operations, and their only source of revenue is from...
-
You are working in a team and your team member asks you to share your credentials so that he can submit his work. He has some trouble with his own account and cannot continue unless you help him....
-
What's a good attention gainer for know your Marines and look out for their welfare?
-
A debt of $3074.83 is due April 1, 2023. What is the value of the obligation on April 1, 2018, if money is worth 3% compounded annually? The value of the obligation is $
-
Among the many campaigns on TikTok, there is a campaign with Pringles. #Playwithpringles is a campaign to create and post videos like popping out of a Pringles barrel. How this campaign get more...
-
The current asset portion of American Eagle Outfitters, Inc.'s balance sheet is as follows (in $000s). Required Prepare a horizontal analysis of American Eagle's current assets, rounding your answers...
-
How can NAFTA be beneficial to suppliers of Walmart?
-
Draw the structure of the product with molecular formula C 10 H 10 O that is obtained when the compound below is heated with aqueous acid. CN CN C10H100 Heat
-
Lactones can be prepared from diethyl malonate and epoxides. Diethyl malonate is treated with a base, followed by an epoxide, followed by heating in aqueous acid: Using this process, identify what...
-
Predict the major product of the following transformation. CO2ET C10H100 Heat
-
Why was early Capital One Alexa capability poorly designed?
-
The North American numbering plan (NANP) specifies the format oftelephone numbers in the U.S., Canada, and many parts of NorthAmerica. A number in this plan consists of ten digits whichare split into...
-
With respect to side agreements involving receivables, an auditor should consider the use of additional audit procedures to test for those which agreements may include ?

Study smarter with the SolutionInn App


