Given the following equilibrium constants at 427C, Determine the values for the equilibrium constants for the following
Question:
Given the following equilibrium constants at 427°C,
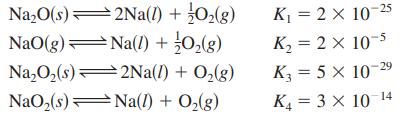
Determine the values for the equilibrium constants for the following reactions:
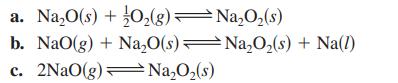
Transcribed Image Text:
2Na(1) + O₂(g) Na₂O(s) NaO(g) Na(1) + O₂(g) Na₂O₂ (s) 2Na(1) + O₂(g) NaO₂(s)Na(l) + O₂(g) K₁ = 2 × 10-25 K₂= 2 × 10-5 K3 = 5 x 10-2 29 K₁ = 3 × 10-14 -
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
To determine the equilibrium constants for the reactions youre ...View the full answer

Answered By

Joseph Ogoma
I have been working as a tutor for the last five years. I always help students to learn and understand concepts that appears challenging to them. I am always available 24/7 and I am a flexible person with the ability to handle a wide range of subjects.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Given the following equilibrium constants at 427oC, Na2O(s) 2Na(l) + 12 O2(g) K1 = 2 10-25 NaO(g) Na(l) + l2 O2(g) K2 = 2 10-5 Na2O2(s) 2Na(l) + O2(g) K3 = 5 10-29 NaO2(s) Na(l) + O2(g) K4 = 3...
-
The following equilibrium constants were determined at 1123 K: Write the equilibrium constant expression KP, and calculate the equilibrium constant at 1123 K for C(s) + CO2(g)--2CO(g) CO(g) + Cl2(g)...
-
The following equilibrium constants have been determined for hydrosulfuric acid at 25°C: Calculate the equilibrium constant for the following reaction at the same temperature: H2S(aq) H(aq)HS...
-
What line of code can you add to disable all logging messages in your program?
-
Lazio never reported the income or paid the sales taxes from the Sylvan Beach pizzeria and never obtained workers compensation insurance for its employees. How might these facts have affected the...
-
A shunt-wound dc motor with the field coils and rotor connected in parallel (Fig) operates from a 120-V dc power line. The resistance of the field windings, RI , is 218 .n. The resistance of the...
-
True or False: If \(\operatorname{IRR}(\mathrm{A})>\operatorname{IRR}(\mathrm{B})\), then \(\operatorname{ERR}(\mathrm{A})>\operatorname{ERR}(\mathrm{B})\).
-
Use the information in Exercise 5-6A to complete the following requirements. In Exercise 5-6A, Terry Industries produces two electronic decoders, P and Q. Decoder P is more sophisticated and requires...
-
One of the driving engines that generate profits within capitalist production is the way that the price of wages is determined. The capitalist pays a wage for the worker that is different than the...
-
The following balance sheets have been prepared as at December 31, Year 6, for Kay Corp. and Adams Ventures: Additional Information: Kay acquired its 40% interest in Adams for $374,000 in Year 2,...
-
For the reaction: K = 1.8 10 -7 at a certain temperature. If at equilibrium [O 2 ] = 0.062 M, calculate the equilibrium O 3 concentration. 30(g) 203(g)
-
At a particular temperature, K = 1.00 10 2 for the reaction In an experiment, 1.00 mole of H 2 , 1.00 mole of I 2 , and 1.00 mole of HI are introduced into a 1.00-L container. Calculate the...
-
Sally purchased a new computer (five-year property) on June 1, 2013, for $4,000. Sally could use the computer 100% of the time in her business, or she could allow her family to use the computer as...
-
Joe and Sam (MFJ) have taxable income of $15,300, tax-exempt income of $25,600, and Social Security benefits of $10,400. What is their provisional income? How much Social Security is taxable?
-
Given the following, calculate profit (enter numbers only, and decimal if applicable). Price: $11 Units Sold: 2400 Fixed Cost: $10000 Variable Cost: $6.5
-
(b) (c) The diagram below shows the routing tables of routers R1, R2, R3 and R4, which connect multiple networks together. Show (in detail) what happens when router R1 receives a packet with...
-
How do I approach an analysis of a case study focusing on Financial Reporting making reference to - Financial Reporting and Regulatory Framework as the main background reading and knowledge.
-
Fred's after tax income is $3600 a month and his expenses are generally $3600 as well. Last month he had a car accident which resulted in a $2000 unexpected expense due to the deductible on his...
-
Marge Simons won a $15 million lottery and elected to receive her winnings in 30 equal installments. After receiving the first 10 installments, Marge and her husband divorced, and the remaining 20...
-
Test whether the 5-year survival rate for breast cancer is significantly different between African American and Caucasian women who are younger than 50 years of age and have localized disease....
-
Pyridine undergoes electrophilic aromatic substitution at the C3 position. Justify this regiochemical outcome by drawing resonance structures of the intermediate produced from attack at C2, at C3,...
-
Predict the product obtained when pyrrole is treated with a mixture of nitric acid and sulfuric acid at 0C.
-
Draw the structure of each of the following compounds: (a) Cyclohexylmethylamine (b) Tricyclobutylamine (c) 2,4-Diethylaniline (d) (1R,2S)-2-Methylcyclohexanamine (e) ortho-Aminobenzaldehyde
-
A company has the demand of 2800 units per year, holding costs of $10 per unit and setup costs (ordering cost) of $30 per order. The demand rate is constant . The order lead time is 1 days, and the...
-
A system contains five tasks. There are three resources X, Y, and Z. The resource requirements of the tasks are specified as follows: T: [X; 1] [Y; 2]. T: [Z; 4]. T: [X; 2]. 3: T: [X, 2][Y; 3]. 4:...
-
First, should US hospitality organizations (hotels, restaurants, events, etc.) go global? Why or why not? Define and discuss the difference between going "multinational" and "transnational." What...

Study smarter with the SolutionInn App


