The vanadium in a sample of ore is converted to VO 2+ . The VO 2+ ion
Question:
The vanadium in a sample of ore is converted to VO2+. The VO2+ ion is subsequently titrated with MnO4- in acidic solution to form V(OH)4+ and manganese(II) ion. The unbalanced titration reaction is
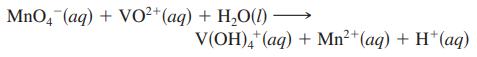
To titrate the solution, 26.45 mL of 0.02250 M MnO4- was required. If the mass percent of vanadium in the ore was 58.1%, what was the mass of the ore sample?
Transcribed Image Text:
MnO4 (aq) + VO²+ (aq) + H₂O(1) - V(OH)4+ (aq) + Mn²+ (aq) + H+ (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To balance the titration reaction we can use the oxidation state method MnO4 aq VO2 aq H2Ol VOH4 aq ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
A 0.5510-g sample consisting of a mixture of iron and iron(III) oxide was dissolved completely in acid to give a solution containing iron(II) and iron(III) ions. A reducing agent was added to convert...
-
The total concentration of Ca2 + and Mg2+ in a sample of hard water was determined by titrating a 0.100-L sample of the water with a solution of EDTA4-.The EDTA4- chelates the two cations: It...
-
The following data are for MarvinMarvin Department Store. The account balances (in thousands) are for 2017LOADING... (Click the icon to view the account balances.) Requirements 1. Compute (a) the...
-
Answer the Multple Choice Questions and the code for problem 6in the end PROBLEM 1: General UNIX 1. What is UNIX? a) an operating system b) a text editor c) programming language d) software program...
-
Observe the operations at your favorite fast-food restaurant. Required 1. How many people does it take to fill a typical order of sandwich, beverage, and one side-order? 2. Describe the activities...
-
For Concept 2, the statement is correct regarding: A. sensitivity analysis, but not correct regarding scenario analysis. B. scenario analysis, but not correct regarding sensitivity analysis. C. both...
-
Based on the design, briefly discuss the data collection procedures to be used. Be sure to include the area rea of focus and targeted sample as part of these procedures. Develop a hypothetical...
-
MKM International is seeking to purchase a new CNC machine in order to reduce costs. Two alternative machines are in consideration. Machine 1 costs $500,000, but yields a 15 percent savings over the...
-
3) The cost and revenue function in dollar terms for a fast-food joint is given as follows: C(x)=200x+200 and R(x) = 50x X + 4 If x is measured in hundreds of units, what is the break-even point for...
-
Laval produces lighting fixtures. Budgeted Information for Its two production departments follows. The departments use machine hours (MH) and direct labor hours (DLH). Overhead cost Direct labor...
-
A 0.500-L sample of H 2 SO 4 solution was analyzed by taking a 100.0-mL aliquot and adding 50.0 mL of 0.213 M NaOH. After the reaction occurred, an excess of OH - ions remained in the solution. The...
-
The blood alcohol (C 2 H 5 OH) level can be determined by titrating a sample of blood plasma with an acidic potassium dichromate solution, resulting in the production of Cr 3+ (aq) and carbon...
-
Refer to the data for Helox Ltd in E5-10. Assume that the company uses the FIFO cost method. Data from E5-10 Helox Ltd manufactures a product that passes through two production processes. A quantity...
-
The Salem Corporation sells inventory costing $15,000 each month during Year 1. At the beginning of the year, the company's balance sheet showed inventory with a balance of $22,000 and accounts...
-
= 1, the pathloss for dif- Consider a channel with normalized noise level ferent subbands PL =0., 13. 20dB, respectively, and total power constraint P 100. Compute the maximal admissible capacity...
-
Green Moose Industries reported sales of $890,000 at the end of last year, but this year, sales are expected to grow by 8%. Green Moose expects to maintain its current profit margin of 20% and...
-
Calculate her taxes paid (ignore net investment income tax) if she elects to exclude preferential rate investment income in "investment income" purposes of calculating investment interest expense ?
-
On August 1, 2020, X offered for sale his two-hectare lot to Z for 10M pesos and gave the latter who showed interest to buy, thirty (30) days within which to decide on the matter. Five days...
-
Rutherford Corporation has 20,000 shares of its $100 par value, 7 percent cumulative preferred stock outstanding and 100,000 shares of its $1 par value common stock outstanding. In Rutherfords first...
-
By referring to Figure 13.18, determine the mass of each of the following salts required to form a saturated solution in 250 g of water at 30 oC: (a) KClO3, (b) Pb(NO3)2, (c) Ce2(SO4)3.
-
The compound Ni(H 2 O) 6 Cl 2 is green, whereas Ni(NH 3 ) 6 Cl 2 is violet. Predict the predominant color of light absorbed by each compound. Which compound absorbs light with the shorter wavelength?...
-
Oxalic acid is often used to remove rust stains. What properties of oxalic acid allow it to do this?
-
A certain first-row transition metal ion forms many different colored solutions. When four coordination compounds of this metal, each having the same coordination number, are dissolved in water, the...
-
Think of a brand they engage with on social media or have. For the discussion talk about how the brand is effective on social media? What do they do well? What types of posts do they make?
-
Part 2, your goal is to complete a marketing plan based onToyota Prius of the Toyota Motor Corporation. The marketing plan is strategically providing directions for what you believe the company...
-
1) search social media sites to get an idea of how the public may perceive the company? 2) learn what you can about the senior executives of the company and their plans?

Study smarter with the SolutionInn App


