A 0.5510-g sample consisting of a mixture of iron and iron(III) oxide was dissolved completely in acid
Question:
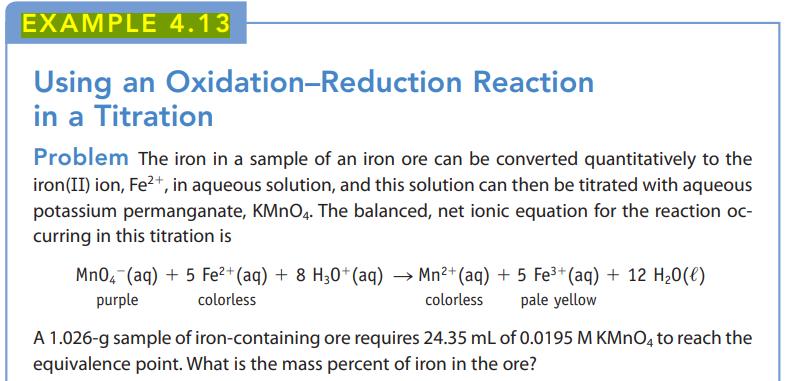
A 0.5510-g sample consisting of a mixture of iron and iron(III) oxide was dissolved completely in acid to give a solution containing iron(II) and iron(III) ions. A reducing agent was added to convert all of the iron to iron(II) ions, and the solution was then titrated with the standardized KMnO4 (0.04240 M); 37.50 mL of the KMnO4 solution was required. Calculate the mass percent of Fe and Fe2O3 in the 0.5510-g sample. (Example 4.13 gives the equation for the reaction of iron(II) ions and KMnO4.)
Data given in Example 4.13
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





