A herbicide contains 2,4-D (2,4-dichlorophenoxyacetic acid), C 8 H 6 Cl 2 O 3 . A 1.236-g
Question:
A herbicide contains 2,4-D (2,4-dichlorophenoxyacetic acid), C8H6Cl2O3. A 1.236-g sample of the herbicide was decomposed to liberate the chlorine as Cl− ion. This was precipitated as AgCl, with a mass of 0.1840 g. What is the mass percent of 2,4-D in the sample?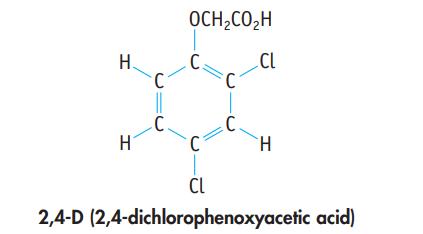
Transcribed Image Text:
H. H C C. OCH₂CO₂H C. C C. CL H CL 2,4-D (2,4-dichlorophenoxyacetic acid)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To find the mass percent of 24D in the sample you need to first calculate the molar mass of 24D and ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
This activity has the purpose of helping students explain the purposes and uses of measures of employee performance and identify the systematic way of integrated components which comprise an overall...
-
Files Links: https://drive.google.com/drive/folders/10CssG86ruVkS1bwe-B7VIpwBx2twS6n-?usp=share_link https://drive.google.com/drive/folders/10CssG86ruVkS1bwe-B7VIpwBx2twS6n-?usp=share_link You will...
-
An airplane typically approaches a runway for landing at a descent angle of 3. However, a jet experiences problems and makes an emergency descent through the air at an angle of 10. If this jet is...
-
Macmillan Learning A Geiger-Muller tube is a type gas-filled radiation detector. It can detect particles like X-rays, alpha particles, and beta rays (electrons). This is useful in quantizing the...
-
Explain the differences among factor rating, the loaddistance model, and break-even analysis. What criteria does each method use to make the location decision?
-
Use the time value of money tables in Exhibit 1 8 to calculate the following: a. The future value of $100 at 7 percent in 10 years. b. The future value of $100 a year for 6 years earning 6 percent....
-
A vertical shaft passes through a bearing and is lubricated with an oil having a viscosity of \(0.2 \mathrm{~N} \cdot \mathrm{s} / \mathrm{m}^{2}\) as shown in Fig. P6.85. Assume that the flow...
-
A supermarket chain wants to know if their buy one, get one free campaign increases customer traffic enough to justify the cost of the program. For each of 10 stores they select two days at random to...
-
Consider an investor who purchases a Treasury inflation-indexed note with an original principal amount of $100,000, a 6.500 percent annual coupon rate (coupon is paid semiannually), and 10 years to...
-
Sulfuric acid is listed in a catalog with a concentration of 9598%. A bottle of the acid in the stockroom states that 1.00 L has a mass of 1.84 kg. To determine the concentration of sulfuric acid in...
-
Thioridazine, C 21 H 26 N 2 S 2 , is a pharmaceutical agent used to regulate dopamine. (Dopamine, a neurotransmitter, affects brain processes that control movement, emotional response, and ability to...
-
Use Definition 7.1.1 to find {f (t)}. f(t) = t cos t Definition 7.1.1 Let f be a function defined for t = 0. Then the integral
-
The beginning work in process inventory was 80% complete for materials and 65% complete for labor and overhead. The ending work in process inventory was 60% complete for materials and 40% complete...
-
Doctor Strange completed its first fundraising campaign in December 2019. It was successful and resulted in pledges totaling $100,000, for use at management's discretion, to be collected in March...
-
7 In 2020, a firm had current assets of $3,000, total assets of $12,000, current liabilities of $4,000, total liabilities of $7,000, and total equity of $5,000 In 2021, the same firm had current...
-
Beresford Incorporated purchased several investments in debt securities during 2023, its first year of operations. The following information pertains to these securities. The fluctuations in their...
-
A cargo plane is moving with a horizontal velocity of vx = + 2 3 1 m / s at a height of y = 1 1 7 0 m above level ground when it releases a package. Ignoring air resistance, how much time, in...
-
What accounts on the balance sheet must be evaluated when completing the financing activities section of the statement of cash flows?
-
Discuss the concept of the looking-glass self. how do you think others perceive you? do you think most people perceive you correctly?
-
Draw the product for each of the following S N 2 reactions: (a) (S)-2-Chloropentane and NaSH (b) (R)-3-Iodohexane and NaCl (c) (R)-2-Bromohexane and sodium hydroxide
-
When (S)-1-bromo-1-fluoroethane reacts with sodium methoxide, an S N 2 reaction takes place in which the bromine atom is replaced by a methoxy group (OMe). The product of this reaction is...
-
Draw the transition state for each of the following S N 2 reactions: (a) (b) (c) (d) Br Br P
-
explain (a) Describe how mutual-exclusion locks provided by the synchronized keyword can be used to control access to shared data structures. In particular you should be clear about the behaviour of...
-
Almost all data transferred over the Internet use the Internet Protocol at the Network layer. Choose any webpage that is freely available via the Internet (it must not require login, signup, etc) and...
-
5.) Using the three coordinates from problem 4, create a parabolic model H(x) in the standard form H(x)=ax+ bx+c for the path of your object where H(x) is the height when the object is x units from...

Study smarter with the SolutionInn App


