(a) Suppose you have a cube of copper metal that is 0.236 cm on a side with...
Question:
(a) Suppose you have a cube of copper metal that is 0.236 cm on a side with a mass of 0.1206 g. If you know that each copper atom (radius = 128 pm) has a mass of 1.055 × 10−22 g (you will learn in Chapter 2 how to find the mass of one atom), how many atoms are there in this cube? What fraction of the cube is filled with atoms? (Or conversely, how much of the lattice is empty space?) Why is there “empty” space in the lattice?
(b) Now look at the smallest, repeating unit of the crystal lattice of copper. 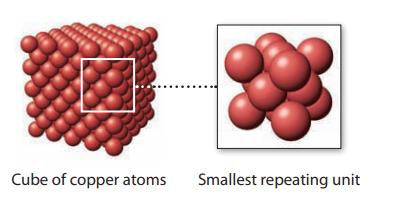
Knowing that an edge of this cube is 361.47 pm and the density of copper is 8.960 g/cm3, calculate the number of copper atoms in this smallest, repeating unit
Step by Step Answer:

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel





