Question: You set out to determine the density of lead in the laboratory. Using a top loading balance to determine the mass and the water displacement
You set out to determine the density of lead in the laboratory. Using a top loading balance to determine the mass and the water displacement method (Study Question 41) to determine the volume of a variety of pieces of lead, you calculate the following densities: 11.6 g/cm3, 11.8 g/cm3, 11.5 g/cm3, and 12.0 g/cm3. You consult a reference book and find that the accepted value for the density of lead is 11.3 g/cm3. Calculate your average value, percent error, and standard deviation of your results.
Data given in Question 41
You have a 250.0-mL graduated cylinder containing some water. You drop three marbles with a total mass of 95.2 g into the water. What is the average density of a marble?
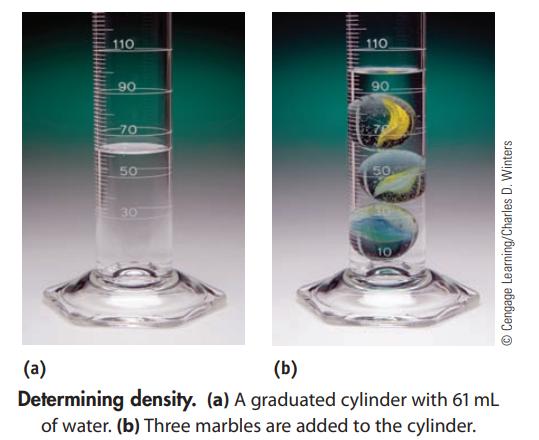
110 90 70 50 30 110 90 50 10 Cengage Learning/Charles D. Winters (a) (b) Determining density. (a) A graduated cylinder with 61 mL of water. (b) Three marbles are added to the cylinder.
Step by Step Solution
3.34 Rating (157 Votes )
There are 3 Steps involved in it
Determine the density of lead average density percent error and standard deviation using the provid... View full answer

Get step-by-step solutions from verified subject matter experts


