A voltaic cell is set up utilizing the reaction Under standard conditions, the expected potential is 0.45
Question:
A voltaic cell is set up utilizing the reaction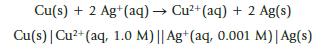
Under standard conditions, the expected potential is 0.45 V. Predict whether the potential for the voltaic cell will be higher, lower, or the same as the standard potential. Verify your prediction by calculating the new cell potential.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





