Answer the following questions as a summary quiz on this chapter. (a) The quantum number n describes
Question:
Answer the following questions as a summary quiz on this chapter.
(a) The quantum number n describes the __________ of an atomic orbital, and the quantum number ℓ describes its __________.
(b) When n = 3, the possible values of ℓ are __________.
(c) What type of orbital corresponds to ℓ = 3?__________
(d) For a 4d orbital, the value of n is __________, the value of ℓ is __________, and a possible value of mℓ is __________.
(e) Each of the following drawings represents a type of atomic orbital. Give the letter designation for the orbital, give its value of ℓ, and specify the number of planar nodes.
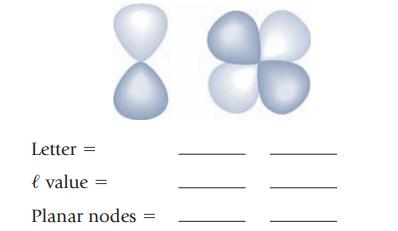 (f) An atomic orbital with three planar nodes through the nucleus is a(n) _______ orbital.
(f) An atomic orbital with three planar nodes through the nucleus is a(n) _______ orbital.
(g) Which of the following orbitals cannot exist according to modern quantum theory: 2s, 3p, 2d, 3f, 5p, 6p?
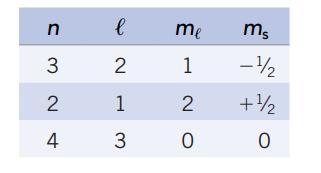
(h) Which of the following is not a valid set of quantum numbers?
 (i) What is the maximum number of orbitals that can be associated with each of the following sets of quantum numbers? (One possible answer is “none.”)
(i) What is the maximum number of orbitals that can be associated with each of the following sets of quantum numbers? (One possible answer is “none.”)
(i) n = 2 and ℓ = 1
(ii) n = 3
(iii) n = 3 and ℓ = 3
(iv) n = 2, ℓ = 1, and mℓ = 0
Step by Step Answer:

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel





