Question: The data in the following table have been obtained for the potential of the cell Pt(s) |H 2 (g, f = 1atm) HCl(aq, m)|AgCl(s) Ag(s)
The data in the following table have been obtained for the potential of the cell Pt(s) |H2(g, f = 1atm) HCl(aq, m)|AgCl(s) Ag(s) as a function of m at 25°C.
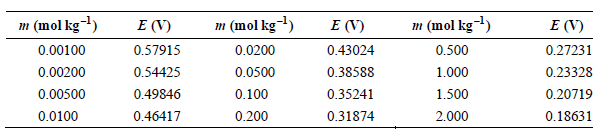
a. Determine E° using a graphical method.
b. Calculate γ ± for HCl at m = 0.00100, 0.0100, and 0.100 mol kg–1.
m (mol kg) m (mol kg) m (mol kg-l) E (V) 0.57915 0.54425 0.49846 E (V) E (V) 0.27231 0.23328 0.20719 0.500 0.00100 0.0200 0.43024 0.38588 0.35241 0.00200 0.0500 1.000 0.00500 0.0100 1.500 2.000 0.100 0.200 0.46417 0.31874 0.18631
Step by Step Solution
3.33 Rating (150 Votes )
There are 3 Steps involved in it
Cell reaction 2AgCls H 2 g2Ags 2H aq 2Cl aq In the low ... View full answer

Get step-by-step solutions from verified subject matter experts


