Assume you have 0.123 mol of each of the following compounds. What mass of each is present?
Question:
Assume you have 0.123 mol of each of the following compounds. What mass of each is present?
(a). C14H10O4, benzoyl peroxide, used in acne medications
(b). Dimethylglyoxime, used in the laboratory to test for nickel(II) ions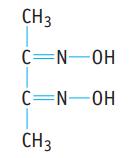
(c). The compound below, responsible for the “skunky” taste in poorly made beer. 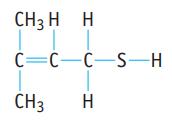
(d). DEET, a mosquito repellent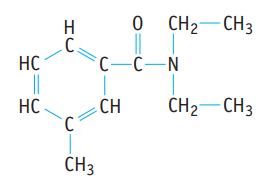
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





