Boron nitride, BN, has the same solid state structure as ZnS (Figure 12.10). Data given in Figure
Question:
Boron nitride, BN, has the same solid state structure as ZnS (Figure 12.10).
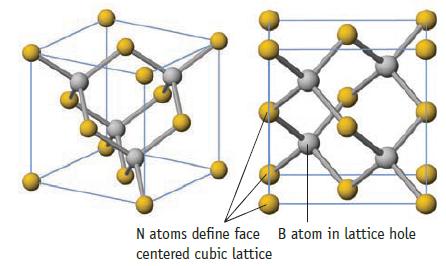
Data given in Figure 12.10
The density of copper metal is 8.95 g/cm3. If the radius of a copper atom is 127.8 pm, is the copper unit cell primitive, body-centered cubic, or face-centered cubic?
Transcribed Image Text:
N atoms define face B atom in lattice hole centered cubic lattice
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Yes that is correct Boron nitride BN and zinc sulfide ZnS have the same solid state struct...View the full answer

Answered By

Giri G
I have completed my BTech in electrical and electronics engineering in 2018 with first class
After that I prepared for GATE and got cleared with pretty decent score. While gate preparation I took classes for my juniors and students near my home town
Right now I am planning for persuing MS abroad. And the process is ongoing.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
The density of copper metal is 8.95 g cm3 If the radius of copper atom is 127.8 pm, is the copper unit cell simple cubic, body centred cubic or face-centred cubic? (At mass of Cu=63.54g mol1 and NA =...
-
The density of copper metal is 8.95 g/cm 3 . If the radius of a copper atom is 127.8 pm, is the copper unit cell primitive, body-centered cubic, or facecentered cubic?
-
A coffee-cup calorimeter of the type shown in Figure 5.18 contains 150.0 g of water at 25.1 C. A 121.0-g block of copper metal is heated to 100.4 C by putting it in a beaker of boiling water. The...
-
9. Let x = (1.11... 111000...) 26, in which the fractional part has 26 1's followed by O's. For the Marc-32, determine x, x+, f(x), x-xx-x, xx, and lx-fl(x)/x.
-
Elijah Electronics makes PDAs. The firm produced 45,000 PDAs during its first year of operation. At year-end, it had no inventory of finished goods. Elijah sold 42,300 units through regular market...
-
Construct second-order transfer functions that meet the following requirements. Use MATLAB to plot the transfer function's Bode diagram and validate the requirements. (rad/s) Low pass 200,000 * T(J...
-
In December 2008, Jason Garcia signed a motor vehicle sales contract with Mac Haik Dodge Chrysler Jeep, a dealer. In the contract, Garcia agreed to purchase a 2009 Dodge Ram 1500. The contract...
-
Cost allocation and decision making. Greenbold Manufacturing has four divisions named after its locations: Arizona, Colorado, Delaware, and Florida. Corporate headquarters is in Minnesota. Greenbold...
-
Bank extends a revolving line of credit to Epstein Corp., secured by Epstein's inventory and receivables. Epstein has now filed bankruptcy. At all times during the 90 days preceding the filing the...
-
An aqueous solution of iron(II) sulfate is paramagnetic. If NH 3 is added, the solution becomes diamagnetic. Why does the magnetism change?
-
For the high-spin complex [Fe(H 2 O) 6 ]SO 4 , identify the following: (a) The coordination number of iron (b) The coordination geometry for iron (c) The oxidation number of iron (d) The number of...
-
Find the fraction of gas molecules whose velocities differ by less than = 1.00% from the value of. (a) The most probable velocity; (b) The root mean square velocity.
-
What is the first question that should be asked when determining the amount of retirement income an employer should provide? a. Should other postretirement income sources be integrated with the...
-
A solid, uniform disk of radius 0.400 m and mass 65.0 kg rolls down a ramp of length 7.50 m that makes an angle of 14.5 with the horizontal. The disk starts from rest from the top of the ramp....
-
The changes organizations are adopting due to the workforce's new demands and the labor market's changes resulting from COVID-19 Instructions: Explains the current state, including the problems firms...
-
How wouldyou use employee surveys to support your retention strategy for this job? Employee surveys can give great insights about what employees think, feel, want,and need.Are there any specific...
-
XYZ Company produces two fishing boats: Clipper and Sportsman. The company does not have detailed information on the cost incurred to manufacture both products. Recently, the company has incurred...
-
Andy Mendoza in Problem 7 is concerned that the demand for his dolls will not exceed the break-even point. He believes he can reduce his initial investment by purchasing used sewing machines and...
-
Should we separate the debt and equity features of convertible debt? Team 1: Pro separation: Present arguments in favor of separating the debt and equity features of convertible debt. Team 2: Against...
-
In an amusement park a large upright convex spherical mirror is facing a plane mirror 10.0 m away. A girl 1.0 m tall standing midway between the two sees herself twice as tall in the plane mirror as...
-
Considering the operation of a spherical mirror, prove that the locations of the object and image are given by - F(Mr 1) s, 3 - fM 1) and Si = So f(M - 1)/ and %3|
-
A device used to measure the radius of curvature of the cornea of the eye is called a keratometer. This is useful information when fitting contact lenses. In effect, an illuminated object is placed a...
-
You will soon be evaluating the strategic plans of the company you chose for the SWOT analysis and the Plan Formulator and Implementer analyses. How do you evaluate the information disclosed by the...
-
The decision of the Ontario Court of Appeal relies upon a variety of sources of law. Identify two sources of law relied upon by the court and identify their weight in decision making. Are they...
-
The world's largest professional project management organization is the Project Management Institute (PMI). Go to their website, www.pmi.org, and examine the links you find. What links suggest that...

Study smarter with the SolutionInn App


