Calculate the value of E for each of the following reactions. Decide whether each is product-favored at
Question:
Calculate the value of E° for each of the following reactions. Decide whether each is product-favored at equilibrium in the direction written. [Reaction (d) is carried out in basic solution.]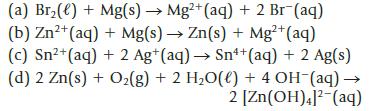
Transcribed Image Text:
(a) Br₂(e) + Mg(s) → Mg2+ (aq) + 2 Br (aq) (b) Zn²+ (aq) + Mg(s) → Zn(s) + Mg²+ (aq) (c) Sn²+ (aq) + 2 Ag+ (aq) → Sn¹+ (aq) + 2 Ag(s) (d) 2 Zn(s) + O₂(g) + 2 H₂O(l) + 4 OH-(aq) → 2 [Zn(OH)4]2 (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To calculate the value of E for each reaction we can use the following equation E Ecathode Eanode wh...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Calculate the value of E for each of the following reactions. Decide whether each is product-favored at equilibrium in the direction written. (a) 2 1 (aq) + Zn+ (aq) 1(s) + Zn(s) (b) Zn+ (aq) +...
-
The compound SbCl5(g) decomposes at high temperatures to gaseous antimony trichloride and chlorine gas. When 89.7 g of SbCl5(g) is placed in a 15.0-L container at 1808C, the SbCl5(g) is 29.2%...
-
The following potential diagram is part of that illustrating the redox chemistry of chlorine in aqueous solution at pH0. (a) Calculate the value of E for the reduction of [ClO 3 ] to HClO 2 . (b)...
-
Food Enterprises is analyzing the performance of their retail business and have calculated Value At Risk at $17,350,000 under the statistical method at a 95% confidence level, and a mean of $898,000....
-
The following data relating to direct materials cost for March of the current year are taken from the records of Top Toys Inc., a manufacturer of plastic toys: Quantity of direct materials used...
-
Continue with the social planner's problem in the Allen-Gale model, Section 2.3. Given the planner's optimal choice on \(c_{1} / c_{2}\) after \(R\) is revealed \(t=1\), solve problem (2.11) for the...
-
Explain the idea behind the BDI architecture. Why do you think this architecture is particularly appealing to human researchers?
-
The Denver advertising agency promoting the new Breem dishwashing detergent wants to get the best exposure possible for the product within the $100,000 advertising budget ceiling placed on it. To do...
-
Sales Variable manufacturing and selling expenses Contribution margin Fixed expenses: Advertising, traceable Depreciation of special equipment Salaries of product-line managers Allocated common fixed...
-
Balance each of the following unbalanced equations; then calculate the standard potential, E, and decide whether each is product-favored at equilibrium as written. (All reactions are carried out in...
-
What reactions occur when a lead storage battery is recharged?
-
The legal specialty concerned with the relationship between businesses or individuals and government agencies. a. administrative law b. appellate court c. civil law d. common law e. criminal law f....
-
The Harvard architecture (Fig. 2.9 ) offers separate address and data buses for instruction codes and data. Why is not it feasible to have separate buses for programmed I/O as well? Figure 2.9 CPU...
-
What are the advantages of systems on chip over computers on chip (Fig. 2.19 )? Find a few examples of commercial systems on chip from the Web. Figure 2.19 Microprocessor Processor Standard...
-
A watchdog timer (Fig. 2.20 ) is used for supervising the operation of an embedded system in a high - EMI environment. Why is it practical to connect the watchdog - circuit s output to the CPU s...
-
Show mathematically that the upper limit for CPU utilization with the rate - monotonic approach, is exactly ln 2 as stated in Equation 3.7 . Equation 3.7 lim n (2-1) 004-1
-
Write save and restore routines in assembly code so that they save and restore the context to/from the head and tail of a ring buffer, respectively instead of using a stack.
-
Sifting, the second department in a three-department production process for Finest Flour Inc., received 10,000 units with a total cost of $25,000 from Milling during May. Production costs in Sifting...
-
The value of a share of common stock depends on the cash flows it is expected to provide, and those flows consist of the dividends the investor receives each year while holding the stock and the...
-
Two mischievous children drop water balloons from a bridge as depicted in Figure P3.87. Each water balloon is approximately 30 cm in diameter, and in this figure the red balloon is about 1.8 m below...
-
An impish young lad stands on a bridge 10 m above a lake and drops a water balloon on a boat of unsuspecting tourists. Although the boat is traveling at a speed of 7.5 m/s, the boy manages to land...
-
A boy pushes a 3.1-kg book against a vertical wall with a horizontal force of 40 N. What is the minimum coefficient of friction that will keep the book in place without sliding?
-
Ozark Distributing Company is primarily engaged in the wholesale distribution of consumer products in the Ozark Mountain regions. The following disclosure note appeared in the company's 2024 annual...
-
Write anLLStack class that implementstheStack interface given below using alinked list rather than an array (as done in class).YourLLStack class should include all themethods in theStack...
-
On September 30, 2024, the Techno Corporation issued 8% stated rate bonds with a face amount of $340 million. The bonds mature on September 30, 2044 (20 years). The market rate of interest for...

Study smarter with the SolutionInn App


