Balance each of the following unbalanced equations; then calculate the standard potential, E, and decide whether each
Question:
Balance each of the following unbalanced equations; then calculate the standard potential, E°, and decide whether each is product-favored at equilibrium as written. (All reactions are carried out in acid solution.)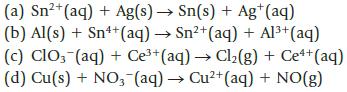
Transcribed Image Text:
2+ (a) Sn²+ (aq) + Ag(s) → Sn(s) + Ag+ (aq) (b) Al(s) + Sn++ (aq) → Sn²+ (aq) + Al³+ (aq) (c) ClO3(aq) + Ce³+ (aq) → Cl₂(g) + Ce++ (aq) (d) Cu(s) + NO3(aq) → Cu²+ (aq) + NO(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To balance these unbalanced redox equations and determine whether they are productfavored at equilib...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Balance each of the following unbalanced equations; then calculate the standard potential, E, and decide whether each is product-favored at equilibrium as written. (All reactions are carried out in...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
A pack of iron bolts is such that the difference in masses or weights of successive sizes is the same bolt being of mass 13.5 grams and the largest is 94.5 grams. If the total mass of the complete...
-
H.J. Heinz Company uses standards to control its materials costs. Assume that a batch of ketchup (1,500 pounds) has the following standards: The actual materials in a batch may vary from the standard...
-
The use of drones has been proposed by retailers like Amazon.com as a method for package delivery. If drones eventually become a widely adopted technology for this purpose, some workers who are...
-
How do you prevent name clashes when using header files?
-
Below are selected T-accounts for the RunnerTech Company. Below are selected T-accounts for the RunnerTech Company. Required: Complete the following journal entries and answer the following...
-
= 1, 2, 3,.... Problem 3.32. Suppose (xi) 1 C R and xi xi+1 for all i Show that there is an x* = (-, 0] such that x converges to x*. That is {x} x*, though perhaps x* .
-
Consider the following half-reactions: (a) Based on E values, which metal is the most easily oxidized? (b) Which metals on this list are capable of reducing Fe 2+ (aq) to Fe(s)? (c) Write a balanced...
-
Calculate the value of E for each of the following reactions. Decide whether each is product-favored at equilibrium in the direction written. [Reaction (d) is carried out in basic solution.] (a)...
-
Using the appropriate definition, (11.44) or (11.45), calculate (x 1 ,x 2 ) for each of the following pairs of signals: (a) e -ItI , 2e -3t u(t) (b) e -(4+j3)t u(t), 2e -(3+j5)t u(t) (e) cos 2t, cos...
-
What is willingness to pay? What does it mean? How is itmeasured (in relation to what standard object from microeconomictheory)? What data do we need? What are limitations of WTP as a concept? Why is...
-
Walmart and Target are duopolist providers of a special vacuum cleaner (you can only buy this vacuum from one of the two firms!) If they both markup the vacuum to a high price of $300, they sell...
-
SpongeBob SquarePants wants to start a business sellingjellyfish as household pets. To get his enterprise started he musttake one day off from work at the Krusty Krab to purchase theneeded equipment...
-
For a ten-year remediation purpose, the City of Avondale, Arizona is purchasing mitigation equipment for $500,000 to pump ground water that will remove contamination and make it potable for...
-
What doesthe term "monetize" mean when speaking about monetarypolicy?
-
The National Western Railroads rail network covers most of the U.S. West and Midwest. On a daily basis it sends empty freight cars from various locations in its rail network to its customers for...
-
Smthe Co. makes furniture. The following data are taken from its production plans for the year. Required: 1. Determine the hazardous waste disposal cost per unit for chairs and for tables if costs...
-
Slap shot. In professional hockey, a slap shot is a type of shot in which a player literally slaps the puck with his stick. The puck may start with a very low speed and can leave the stick with a...
-
A spring scale indicates that a helium balloon tied to it produces a tension of 0.20 N in the string. The string is then cut, and the balloon rises until it comes to rest on the ceiling. (a) Draw a...
-
A subway train is designed with a maximum acceleration of +0.20 m/s 2 , which allows for both passenger safety and comfort. (a) If subway stations are 1.2 km apart, what is the maximum velocity that...
-
Videos for this assignment are found in the Instructions of Child Observation at the beginning of this Module. all 6 videos. Child: Seb (use videos provided in this module) Age: Seb 3.7 years old...
-
please read the instructions and Write a Java program that does the following: 1) At the beginning of your program, include comments that say: MyTest1 implements an application that displays four...
-
I. CONVERTING ASSETS TO EXPENSES 1. On 10/1/20 ABC Corp. purchases, on account, $12,000 of shop supplies to be used during the period 10/1/20 - 9/30/21. ABC Corp. adjusts its books annually. a....

Study smarter with the SolutionInn App


