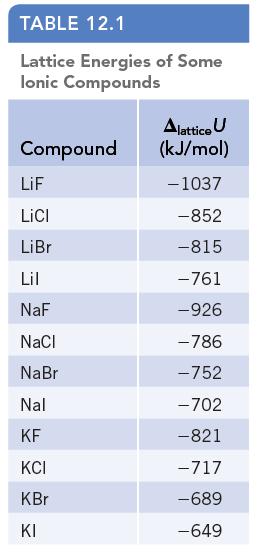
Examine the trends in lattice energy in Table 12.1. The value of the lattice energy becomes somewhat
Question:
Examine the trends in lattice energy in Table 12.1. The value of the lattice energy becomes somewhat more negative on going from NaI to NaBr to NaCl, and all are in the range from −700 to −800 kJ/mol. Suggest a reason for the observation that the lattice energy of NaF (ΔlatticeU = −926 kJ/mol) is much more negative than those of the other sodium halides.
Data given in Table 12.1
Transcribed Image Text:
TABLE 12.1 Lattice Energies of Some Ionic Compounds Compound LiF LICI LiBr Lil NaF NaCl NaBr Nal KF KCI KBr KI Alattice U (kJ/mol) -1037 -852 -815 -761 -926 -786 -752 -702 -821 -717 -689 -649
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
The lattice energy of an ionic compound is a measure of the energy required to separate the ions in a crystal lattice to an infinitely large separatio...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
The kinetic data for the radical chain chlorination of several cycloalkanes (see the adjoining table) illustrate that the C-H bonds of cyclopropane and, to a lesser extent, cyclobutane are somewhat...
-
The critical temperature (K) and pressure (atm) of a series of halogenated are as follows: (a) List the intermolecular forces that occur for each compound. (b) Predict the order of increasing...
-
Addie's grandfather has left them a trust and the value of the money they receive depends on the age choose to collect this gift of money. Their options are given in the table below. Payoff at age 19...
-
You are a management consultant and have been asked by Messrs. Schmidt, Page, and Brin to investigate the public perception of Google as unresponsive, self-centered, and dangerously cocky. How would...
-
Your company is considering the introduction of a new product line. The initial investment required for this project is $500,000, and annual maintenance costs are anticipated to be $45,000. Annual...
-
Samples of groundwater were taken from 5 different toxic-waste dump sites by each of 3 different agencies: the EPA, the company that owned each site, and an independent consulting engineer. Each...
-
Kathryn Shoemaker established Grandmothers Chicken Restaurant in Middleburg five years ago. It features a unique recipe for chicken,just like grandmother used to make. The facility is homey, with...
-
Jason launched a new ad and it has brought an influx of customers to his lawn mowing business. He has a limited amount of employees and wants to make sure he can handle all the new work before...
-
Predict the trend in lattice energy, from least negative to most negative, for the following compounds based on the ion charges and ionic radii: LiI, LiF, CaO, RbI.
-
Potassium iodide has a face-centered cubic unit cell of iodide ions with potassium ions in octahedral holes. The density of KI is 3.12 g/cm 3 . What is the length of one side of the unit cell?
-
Design the logic for a program that outputs numbers in reverse order from 25 down to 0.
-
An agent has to make a choice between two different gambles: a) receive 25000 euro with certainty; b) receive 32000 euro with probability 0.2, 10000 euro with probability 0.7 and 1000 euro with...
-
Are the economies that became member states of the European Union after 2004 catching up to the older members? The file EUGDP2017 contains real GDP growth rates in 2017 for the \(28 \mathrm{EU}\)...
-
In Problem 13.8 on page 526, you used the number of Internet users to predict the number of Facebook users by country (stored in Internet ). Based on these results evaluate whether the assumptions of...
-
In Problem 13.5, you used the GPA to predict the overall satisfaction of university students (stored in UPFBE ). a. At the 0.05 level of significance, is there evidence of a linear relationship...
-
In Problem 13.8, you used the number of Internet users to predict the number of Facebook users by country (stored in Internet ). a. Construct a 95% confidence interval estimate of the mean number of...
-
Explain the usefulness of the consolidated financial statements of a parent corporation for the following stakeholders : a. shareholder of the parent corporation b. major supplier of one of the...
-
Audrey purchases a riding lawnmower using a 2-year, no-interest deferred payment plan at Lawn Depot for x dollars. There was a down payment of d dollars and a monthly payment of m dollars. Express...
-
The 6-in.-diameter L-2 steel shaft on the turbine is supported on journal bearings at A and B. If C is held fixed and the turbine blades create a torque on the shaft that increases linearly from zero...
-
The A-36 hollow steel shaft is 2 m long and has an outer diameter of 40 mm. When it is rotating at 80 rad/s, it transmits 32 kW of power from the engine E to the generator G. Determine the smallest...
-
The A-36 solid steel shaft is 3 m long and has a diameter of 50 mm. It is required to transmit 35 kW of power from the engine E to the generator G. Determine the smallest angular velocity of the...
-
Explain the rationale to enter a mature market with a successful and established brand, the GustBuster. Has Senz turned its back on regional and national governments by manufacturing in China? The...
-
Companies that adopt cloud-based ERP system sacrifice their control over a strategic IT resource. What role would the organization's mission statement play in determining the strategy in acquiring...
-
1). Multinational companies may be less risky than domestic companies. Do you understand that this is or is not correct? Support your answer. 20 points 2) The internationalization process tends to...

Study smarter with the SolutionInn App


