For the reaction BaCO 3 (s) BaO(s) + CO 2 (g), r G = +219.7
Question:
For the reaction BaCO3(s) → BaO(s) + CO2(g),
ΔrG° = +219.7 kJ/mol-rxn. Using this value and other data available in Appendix L, calculate the value of Δf G° for BaCO3(s).
Data given in Appendix L
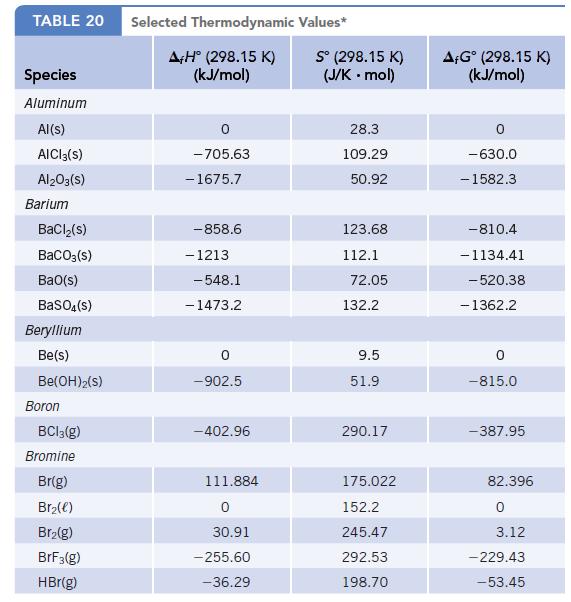
Transcribed Image Text:
TABLE 20 Species Aluminum Al(s) AICI 3(S) Al2O3(S) Barium BaCl₂(s) BaCO3(s) BaO(s) BaSO4(s) Beryllium Be(s) Be(OH)2(S) Boron BC13(g) Bromine Br(g) Br₂(e) Br₂(g) BrF3(g) HBr(g) Selected Thermodynamic A+H° (298.15 K) (kJ/mol) -705.63 - 1675.7 -858.6 - 1213 -548.1 -1473.2 0 -902.5 -402.96 111.884 0 30.91 -255.60 -36.29 Values* Sº (298.15 K) (J/K.mol) 28.3 109.29 50.92 123.68 112.1 72.05 132.2 9.5 51.9 290.17 175.022 152.2 245.47 292.53 198.70 AFGᵒ (298.15 K) (kJ/mol) -630.0 - 1582.3 -810.4 -1134.41 -520.38 - 1362.2 0 -815.0 -387.95 82.396 0 3.12 -229.43 -53.45
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
BaCO3s BaOs CO2g AH for BaCO30 1213 KJmol 30 AH for Baos 5481 KJmol AH for CO2g 39...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
For the reaction TiCl 2 (s) + Cl 2 (g) TiCl 4 (), r G = 272.8 kJ/mol-rxn. Using this value and other data available in Appendix L, calculate the value of f G for TiCl 2 (s). Data given in Appendix...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Calculation of H rxn for Part I 0 1. Assume the heat capacity of the final solution is 4.184 J K-1 g-1. Using the final mass of the solution in the calorimeter, calculate q contents from equation...
-
DS Unlimited has the following transactions during August. August 6 Purchases 88 handheld game devices on account from GameGirl, Inc., for $290 each, terms 1/10, n/60. August 7 Pays $490 to Sure...
-
What advice would you give Michelle about the ethical issues involved in her project? Michelle had been a student union welfare officer on a years sabbatical from her undergraduate accounting and...
-
One of the challenges business owners have is determining how closely to stick to their business plan once the business is launched and they start receiving customer feedback. In almost all cases,...
-
Water boils at \(100^{\circ} \mathrm{C}\). Here, \(100^{\circ} \mathrm{C}\) is the (a) Saturation temperature (b) Boiling point (c) Neither (a) nor (b) (d) Both (a) and (b).
-
Blumen Textiles Corporation began January with a budget for 90,000 hours of production in the Weaving Department. The department has a full capacity of 100,000 hours under normal business conditions....
-
Bill is a 32-year-old male who has worked as an EMT and fireman for most of the last 10 years. He tells you that he can't sleep because he has intrusive dreams. The more he talks to you, the more he...
-
Determine whether the reactions listed below are entropy-favored or disfavored under standard conditions. Predict how an increase in temperature will affect the value of r G. (a) N 2 (g) + 2 O 2 (g)...
-
Using values of f H and S, calculate the standard molar free energy of formation, f G, for each of the following: (a) Ca(OH) 2 (s) (b) Cl(g) (c) Na 2 CO 3 (s) Compare your calculated values of f G...
-
Fill in the unknowns (in thousands of euros): Sales Expenses excluding depreciation Depreciation Total expenses Income before income taxes Income taxes at 30% Net income Cash effects of operations...
-
You deposited $25,000 four years ago into a bank account. Two years ago, you deposited an additional $20,473.39. Assume an annual interest rate of 6% and annual compounding. How much will be in the...
-
You have a credit card balance of $4,300 and are able to make payments of $125 per month. Assume the credit card company charges an annual interest rate of 22%. How many months will it take to pay...
-
Nokomis corporate bonds mature in 10 years, have a par value of $1,000, and have an annual coupon rate of 6.25% (paid on an annual basis). The current market interest rate for comparable bonds is...
-
You are a Human Resource Manager, and your company is expanding globally to Japan from the US. You have been tasked to create a presentation to present to the Executive Leadership Team that reviews...
-
A baseball is thrown from the top of a building at a speed of 29 m / s with 54 of launch angle. The launch height is 20 m. Assume that air resistance is negligible. Find the baseball's final speed...
-
Multiple Choice Questions 1. Which of the following is least likely to be classified as a citys general capital assets? a. Roads and bridges b. Electric utility lines c. Computers used by the police...
-
Pearson Education, a publisher of college textbooks, would like to know if students prefer traditional textbooks or digital textbooks. A random sample of students was asked their preference and the...
-
Atomic emission experiments of a mixture show a calcium line at 422.673 nm corresponding to a 1 P 1 1 S 0 transition and a doublet due to potassium 2 P 3/2 2 S 1/2 and 2 P 1/2 2 S 1/2 transitions...
-
Consider the 1s np 3 P 1s nd 3 D transition in He. Draw an energy-level diagram, taking the spin-orbit coupling that splits terms into levels into account. Into how many levels does each term split?...
-
Calculate the transition dipole moment, for a transition from the 1s level to the 2p z level in H. Show that this transition is allowed. The integration is over r, θ, and . Use for the...
-
Explain the Following Questions: 1. What essential characteristics exist in a proper understanding of "personal mastery," so that as an individual achieves greater progress in this discipline, they...
-
The unknown block (x) in the diagram below is stationary but it is on the verge of moving down the ramp. The coefficient of static friction on the ramp is 0.20. 30 x x Calculate the tension in the...
-
a) What is leadership? What does it mean? Who do you envision when you think of a leader? What does a typical leader do? b) What is management? What does it mean? Who do you envision when you think...

Study smarter with the SolutionInn App


