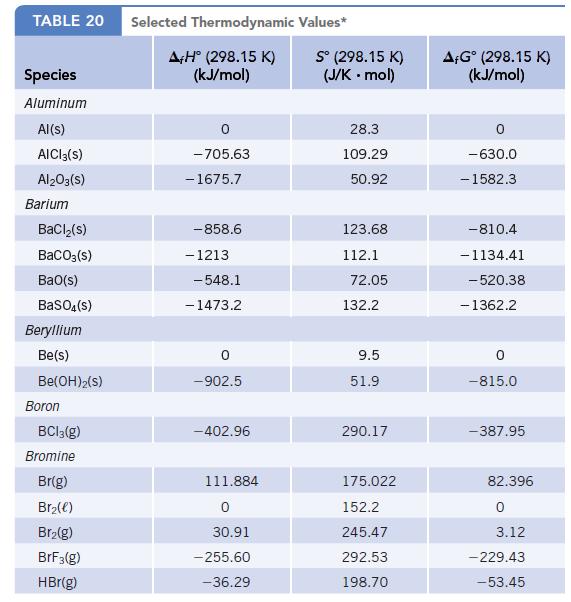
Using values of f H and S, calculate the standard molar free energy of formation,
Question:
Using values of Δf H° and S°, calculate the standard molar free energy of formation, Δf G°, for each of the following:
(a) Ca(OH)2(s)
(b) Cl(g)
(c) Na2CO3(s)
Compare your calculated values of Δf G° with those listed in Appendix L. Which of these formation reactions are predicted to be product-favored at equilibrium at 25°C?
Data given in Appendix L
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





