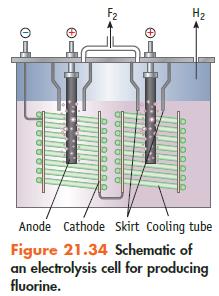
If an electrolytic cell for producing F 2 (Figure 21.34) operates at 5.00 10 3 A
Question:
If an electrolytic cell for producing F2 (Figure 21.34) operates at 5.00 × 103 A (at 10.0 V), what mass of F2 can be produced per 24-hour day? Assume the conversion of F− to F2 is 100%.
Data given in Figure 21.34
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





