Question: In the gas phase, acetic acid exists as an equilibrium of monomer and dimer molecules. (The dimer consists of two molecules linked through hydrogen bonds.)
In the gas phase, acetic acid exists as an equilibrium of monomer and dimer molecules. (The dimer consists of two molecules linked through hydrogen bonds.)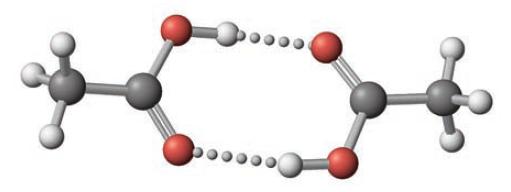
The equilibrium constant, Kc, at 25°C for the monomer–dimer equilibrium![]()
has been determined to be 3.2 × 104. Assume that acetic acid is present initially at a concentration of 5.4 × 10−4 mol/L at 25°C and that no dimer is present initially.
(a) What percentage of the acetic acid is converted to dimer?
(b) As the temperature increases, in which direction does the equilibrium shift? (Recall that hydrogen-bond formation is an exothermic process.)
Step by Step Solution
3.45 Rating (152 Votes )
There are 3 Steps involved in it
a To find the percentage of acetic acid converted to dimer you can set up an ICE Initial Change Equi... View full answer

Get step-by-step solutions from verified subject matter experts


