In the photographic developing process, silver bromide is dissolved by adding sodium thiosulfate. If you want to
Question:
In the photographic developing process, silver bromide is dissolved by adding sodium thiosulfate. 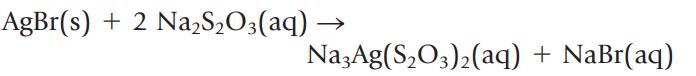
If you want to dissolve 0.225 g of AgBr, what volume of 0.0138 M Na2S2O3, in milliliters, should be used?
Transcribed Image Text:
AgBr(s) + 2 Na,S,O3(aq) Na3Ag(S₂O3)₂(aq) + NaBr(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To calculate the volume of 00138 M Na2S2O3 needed to dissolve 0225 g of AgBr we can use the foll...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
The newspaper production process has come a long way from the old days when the paper was written, edited, typeset and ultimately printed in one building with the journalists working on the upper...
-
Please help with the discusin questions ! I give thumbs up Case #1: Hailing a New Era: Haier in Japan As one of the most valuable brands in China, Haier designs,manufactures, and sells various home...
-
You have $200 and are thinking about betting on the Big Game next Saturday. Your team, the Golden Boars, are scheduled to play their traditional rivals the Robber Barons. It appears that the going...
-
Gabriele Enterprises has bonds on the market making annual payments, with seven years to maturity, a par value of $1,000, and selling for $974. At this price, the bonds yield 7.2 percent. What must...
-
Explain the use of p-charts and c-charts. When would you use one rather than the other? Give examples of measurements for both p-charts and c-charts.
-
Your employer, a mid-sized human resources management company, is considering expansion into related fields, including the acquisition of Temp Force Company, an employ mentagency that supplies word...
-
The following system of differential equations arises in the modeling of a stirred tank reactor with an autocatalyic reaction: \[\begin{aligned}& \frac{d x}{d t}=x-x y \\& \frac{d y}{d t}=-y+x...
-
Was Jay Cohens conviction justified?
-
What are four common mistakes in managing cash? . What are some advantages and disadvantages of using online banking? #3.Compare and contrast a bank with a credit union. #4 What is an advantage and...
-
You can dissolve an aluminum soft drink can in an aqueous base such as potassium hydroxide. If you place 2.05 g of aluminum in a beaker with 185 mL of 1.35 M KOH, will any aluminum remain? What mass...
-
Hydrazine, N 2 H 4 , a base like ammonia, can react with sulfuric acid. What mass of hydrazine reacts with 250. mL of 0.146 M H 2 SO 4 ? 2 NH(aq) + HSO4(aq) 2 NH5+ (aq) + SO4- (aq)
-
In Problems 2326, find the absolute maximum and absolute minimum of each function on the given interval. f(x) = In x on [1, 2]
-
Which management thinker has given the following definition of management: 'The use of people and other resources to accomplish objectives ?
-
Using the cost-minimization model, show what happens when the cost of labor, the wage rate, declines. Correctly label all parts of the graph and identify the changes in labor demand associated with...
-
QUESTION #2 Today, the renovation of Donald's restaurant has been completed. Donald calculates that the renovation and other related expenses total $24,000 on the completion date (i.e. today). He...
-
16. Read each brief description of a project in the following table and classify its type according to the Obeng model (Fog, Quest, Movie, Paint by Numbers (PBN)). (5) Project Reduce the cost of...
-
Capital Structure Project - W. M. Wrigley Jr.(10pts * 6 = 60 pts) For this project, please use Excel to do your calculations and analysis and then type a report in MS Word. Your report should clearly...
-
Use the values in the cross-tabulation table to solve the equations given. a. P(G | A) = _____ b. P(B | F) = _____ c. P(C | E) = _____ d. P(E | G)=_____ A 15 12 8 B 117 19 C 2132 27 D|18|13|12
-
Consider the setup in Problem 16. Show that the relative speed of the ball and the point of contact on the stick is the same before and immediately after the collision. (This result is analogous to...
-
The average heat evolved by the oxidation of foodstuffs in an average adult per hour per kilogram of body weight is 7.20 kJ kg 1 hr 1 . Assume the weight of an average adult is 62.0 kg. Suppose the...
-
Calculate S, S total , and S surroundings when the volume of 150. g of CO initially at 273 K and 1.00 bar increases by a factor of two in a. An adiabatic reversible expansion b. An expansion against...
-
The maximum theoretical efficiency of an internal combustion engine is achieved in a reversible Carnot cycle. Assume that the engine is operating in the Otto cycle and that C V ,m = 5/2 R for the...
-
Determine the ending balance of each of the following T-accounts. Accounts Payable Cash 200 150 3,000 400 160 4,500 120 Supplies 11,000 2,100 11,000 Accounts Receivable 4,800 1,100 250 250 250 200...
-
The following information provides the amount of cost incurred in March for the cost items indicated. During March, 8,100 units of the firm's single product were manufactured. Raw materials Factory...
-
A department of Delta Company incurred the following costs for the month of June. Variable costs, and the variable portion of mixed costs, are a function of the number of units of activity: Activity...

Study smarter with the SolutionInn App


