You can dissolve an aluminum soft drink can in an aqueous base such as potassium hydroxide. If
Question:
You can dissolve an aluminum soft drink can in an aqueous base such as potassium hydroxide.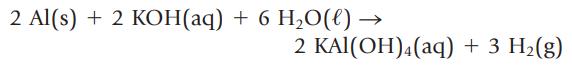
If you place 2.05 g of aluminum in a beaker with 185 mL of 1.35 M KOH, will any aluminum remain? What mass of KAl(OH)4 is produced?
Transcribed Image Text:
2 Al(s) + 2 KOH(aq) + 6 H₂O(l) → 2 KAl(OH)4 (aq) + 3 H₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To determine whether any aluminum remains and to calculate the mass of KAlOH4 produced we can use st...View the full answer

Answered By

Gabriela Rosalía Castro
I have worked with very different types of students, from little kids to bussines men and women. I have thaught at universities, schools, but mostly in private sessions for specialized purpuses. Sometimes I tutored kids that needed help with their classes at school, some others were high school or college students that needed to prepare for an exam to study abroud. Currently I'm teaching bussiness English for people in bussiness positions that want to improve their skills, and preparing and ex-student to pass a standarized test to study in the UK.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
1. What segment of the external environment has more impact on an organization? 2. If an organization wants to engage in a new industry, which area of the industry environment should be the most...
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
Given that log (2) 0.91 and log (5) 2.1, evaluate each of the following. Hint: use the properties of logarithms to rewrite the given logarithm in terms of the the logarithms of 2 and 5. a) log(0.4)~...
-
Explain what is meant by process capability. Why is it important? What does it tell us? How can it be measured?
-
Consider photons emitted from an ultraviolet lamp and a TV transmitter. Which has the greater (a) Wavelength, (b) Energy, (c) Frequency, and (d) Momentum?
-
The stream function for an incompressible flow field is given by the equation \[ \psi=3 x^{2} y-y^{3} \] where the stream function has the units of \(\mathrm{m}^{2} / \mathrm{s}\) with \(x\) and...
-
Multiple Choice Questions The following questions deal with materiality. Choose the best response. a. Which one of the following statements is correct concerning the concept of materiality? (1)...
-
In multi-site manufacturing operations, what role does TPM play in standardizing maintenance practices and ensuring consistency across different plants or facilities? Discuss the challenges and...
-
What volume of 0.750 M Pb(NO 3 ) 2 , in milliliters, is required to react completely with 1.00 L of 2.25 M NaCl solution? The balanced equation is Pb(NO3)2(aq) + 2 NaCl(aq) PbCl(s) + 2 NaNO3(aq)
-
In the photographic developing process, silver bromide is dissolved by adding sodium thiosulfate. If you want to dissolve 0.225 g of AgBr, what volume of 0.0138 M Na 2 S 2 O 3 , in milliliters,...
-
Calculate cash return on assets for 20x8 using the following data: A company has net cash flows from operating activities of $3,000 in 20x8, beginning total assets of $26,000, and ending total assets...
-
Your friend currently has no savings, but wants to accumulate $ 1 million by depositing $ 1 , 5 5 9 per month into an account starting 1 month from today. How long ( in months ) will it take your...
-
An investment is being evaluated that is expected to provide monthly cash flows starting 1 2 months from today, and then lasting forever. The first cash flow is expected to be $ 5 4 1 and the...
-
Routine road maintenance can be done at intervals of 1, 2, 3, 4, or 5 years, but the longer the interval, the higher the cost. Maintenance costs per mile are estimated as $80,000, $170,000, $285,000,...
-
What is Amazon's WACC? Note: Amazon does not have r preferred stock. Apply the CAPM to estimate the cost of equity using the expected market return and risk-free rate from the Walmart WACC estimate....
-
We will be looking into the the Economics of Specialty Choice Among Medical Graduates in the United States. Find 4-5 scholarly references and produce an economic analysis and analyze the net present...
-
What is the expense account associated with the cost of uncollectible receivables called?
-
Using Apple, demonstrate how the differentiation strategy can be well implemented.
-
2.25 moles of an ideal gas with C V ,m = 3/2 R undergoes the transformations described in the following list from an initial state described by T = 310.K. and P = 1.00 bar. Calculate q, w, U, H, and...
-
Compounds A, B, C, and D are constitutionally isomeric, aromatic compounds with molecular formula C 8 H 10 . Deduce the structure of compound D using the following clues. The 1 H NMR spectrum of...
-
Consider the reversible Carnot cycle shown in Figure 5.2 with 1.25 mol of an ideal gas with C V = 5/2R as the working substance. The initial isothermal expansion occurs at the hot reservoir...
-
On November 1, 20X1, an accrual basis taxpayer receives $3,600 in advance for 24-months of subscription services. The taxpayer includes in gross income _____________ in 20X1, _____________ in 20X2...
-
When making a resume, I want to divide my contact information into 3 sections, but cannot remember what tool to use. What tool should she use?
-
What of he following areas is not a target of countermeasure activities found in a lean approach to managing processes?

Study smarter with the SolutionInn App


