Magnesium metal is oxidized, and silver ions are reduced in a voltaic cell using Mg 2+ (aq,
Question:
Magnesium metal is oxidized, and silver ions are reduced in a voltaic cell using Mg2+(aq, 1 M) | Mg and Ag+(aq, 1 M) | Ag half-cells.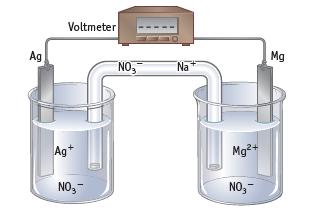
(a) Label each part of the cell.
(b) Write equations for the half-reactions occurring at the anode and the cathode, and write an equation for the net reaction in the cell.
(c) Trace the movement of electrons in the external circuit. Assuming the salt bridge contains NaNO3, trace the movement of the Na+ andNO3− ions in the salt bridge that occurs when a voltaic cell produces current. Why is a salt bridge required in a cell?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





