Methane, CH 4 , can be converted to methanol, which, like ethanol, can be used as a
Question:
Methane, CH4, can be converted to methanol, which, like ethanol, can be used as a fuel. The energy level diagram shown here presents relationships between energies of the fuels and their oxidation products. Use the information in the diagram to answer the following questions. (The energy terms are per mol-rxn.)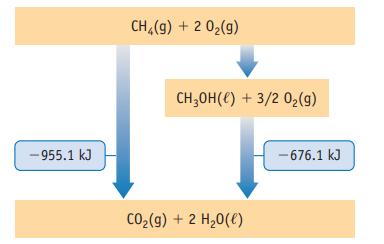
(a) Which fuel, methanol or methane, yields the most energy per mole when burned?
(b) Which fuel yields the most energy per gram when burned?
(c) What is the enthalpy change for the conversion of methane to methanol by reaction with O2(g)?
(d) Each arrow on the diagram represents a chemical reaction. Write the equation for the reaction that converts methane to methanol.
Step by Step Answer:

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel





