Oxidation of 1.00 g of carbon monoxide, CO, produces 1.57 g of carbon dioxide, CO 2 .
Question:
Oxidation of 1.00 g of carbon monoxide, CO, produces 1.57 g of carbon dioxide, CO2. How many grams of oxygen were required in this reaction?
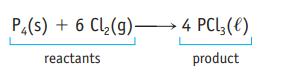
Transcribed Image Text:
P4(s) + 6 Cl₂(g) 4 PCL3 (1) reactants product
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To answer the question we can use the following balanced chemical equation for the ...View the full answer

Answered By

Aqib Parvej
I am teaching since my graduation time so I have teaching experience of about 5 years and in these years I learn to teach in the best and interesting way .
4.80+
20+ Reviews
41+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
How is decision tree analysis used in business analytics? Give an example of how it is used to solve a business problem.
-
Lime (calcium oxide) is widely used in the production of cement, steel, medicines, insecticides, plant and animal food, soap, rubber, and many other familiar materials. It is usually produced by...
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
A common stock pays an annual dividend that increases by 4% annually and sells for $35 per share. If the market rate of return on this stock is 8%. What is the amount of the ?last dividend paid $1.53...
-
Suppose that demand and supply are exactly as described in problem 3 but that there is no marginal social benefit to production. However, for political reasons the government counts a dollars worth...
-
The following income statement, statement of cash flows, and additional information are available for PEK Company: Additional information: a. Beginning inventory and purchases for the one product the...
-
A hydraulic press has a plunger diameter 20 mm and the ram diameter 160 mm. The stroke of the plunger is 200 mm, and the weight lifted by the press is 10 kN. The distance travelled by the weight is...
-
Skie Incorporated had the following transactions involving current assets and current liabilities during February 2012. Feb. 3 Collected accounts receivable of $15,000. 7 Purchased equipment for...
-
How are best fit and best practice HR practices similar and different, what are the benefits and limitations of each?
-
A 0.20 mol sample of magnesium burns in air to form 0.20 mol of solid MgO. What amount (moles) of oxygen (O 2 ) is required for a complete reaction? P4(s) + 6 Cl(g) 4 PCL3 (1) reactants product
-
The equation for the reaction of aluminum and bromine is If you use 6.0 10 23 molecules of Br 2 in a reaction how many atoms of Al will be consumed? 2 Al(s) + 3 Br(l) AlBro(s)
-
Using the fact that |z1 z2| is the distance between two points z1 and z2, give a geometric argument that (a) |z 4i| + |z + 4i| = 10 represents an ellipse whose foci are (0, 4); (b) |z 1| = |z +...
-
(1) What is an information system ? (2) What is the data ? (3) What are activities produce information that organizations need ? (4) What are the information technology dimension of information...
-
A local population of bears starts off with zo 100 members and grows at the rate of 10% per year. (a) (1 point) Write down the bear populations for the first six years, starting with the initial...
-
It is known that an electron with effective mass (m=0.2m0) is inside a practically infinite potential well of 14 nm in width. What is the energy of its first bound state in units of eV? Hint:...
-
Flax Corp. uses the direct method to prepare its statement of cash flows. Flax's trial balances at December 31 for Year 6 and Year 5 show the following information: Account Cash Accounts Receivable...
-
A borrower takes out a 30-year fixed rate mortgage for $310,000 and a rate of 4.1%. Five years later, rates have fallen and the borrower can now obtain a new 30-year mortgage, refinancing the...
-
A parent company acquired an 80% interest in a subsidiary on January 1, 2011, at a price high enough to result in goodwill. Included in the assets of the subsidiary are inventory with a book value of...
-
Quality Chicken grows and processes chickens. Each chicken is disassembled into five main parts. Information pertaining to production in July 2012 is: Joint cost of production in July 2012 was $50. A...
-
Calculate H and U for the transformation of 2.50 mol of an ideal gas from 19.0C and 1.00 atm to 550.C and 19.5 atm if C P,m = 20.9 + 0 042 T/K in units of J K -1 mol -1 .
-
A 1.75 mole sample of an ideal gas for which C V ,m = 20.8 J K -1 mol -1 is heated from an initial temperature of 21.2C to a final temperature of 380.C at constant volume. Calculate q, w, U, and H...
-
An ideal gas undergoes a single-stage expansion against a constant external pressure P external = P f at constant temperature from T,P i ,V i , to T,P f ,V f . a. What is the largest mass m that can...
-
Evaluate how well HRM initiatives contribute to organizational goals and pinpoint any elements that can obstruct their accomplishment.
-
addi x2, x0, 16 slli x2, x2, 4 What is the value of x2 in decimal after the execution?
-
Write a C program that uses the random number generator rand( ) to create an array with 20 numbers in the range from 1 to 50 and disply them in a row. The program sorts the array elements and...

Study smarter with the SolutionInn App


