The cancer drug cisplatin, Pt(NH 3 ) 2 Cl 2 , can be made by reacting (NH
Question:
The cancer drug cisplatin, Pt(NH3)2Cl2, can be made by reacting (NH4)2PtCl4 with ammonia in aqueous solution. Besides cisplatin, the other product is NH4Cl.
(a) Write a balanced equation for this reaction.
(b) To obtain 12.50 g of cisplatin, what mass of (NH4)2PtCl4 is required? What volume of 0.125 M NH3 is required?
(c) Cisplatin can react with the organic compound pyridine, C5H5N, to form a new compound.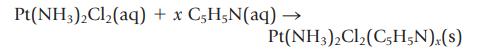
Suppose you treat 0.150 g of cisplatin with what you believe is an excess of liquid pyridine (1.50 mL; d = 0.979 g/mL). When the reaction is complete, you can find out how much pyridine was not used by titrating the solution with standardized HCl. If 37.0 mL of 0.475 M HCl is required to titrate the excess pyridine, ![]() what is the formula of the unknown compound Pt(NH3)2Cl2(C5H5N)x?
what is the formula of the unknown compound Pt(NH3)2Cl2(C5H5N)x?
Step by Step Answer:

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel





