You place 2.56 g of CaCO 3 in a beaker containing 250. mL of 0.125 M HCl.
Question:
You place 2.56 g of CaCO3 in a beaker containing 250. mL of 0.125 M HCl. When the reaction has ceased, does any calcium carbonate remain? What mass of CaCl2 can be produced?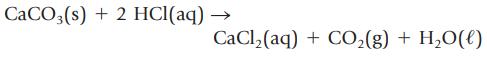
Transcribed Image Text:
CaCO3(s) + 2 HCl(aq) → CaCl₂(aq) + CO₂(g) + H₂O(l)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
To determine if any calcium carbonate CaCO3 remains and to calculate the mass of calcium chloride Ca...View the full answer

Answered By

Madhvendra Pandey
Hi! I am Madhvendra, and I am your new friend ready to help you in the field of business, accounting, and finance. I am a College graduate in B.Com, and currently pursuing a Chartered Accountancy course (i.e equivalent to CPA in the USA). I have around 3 years of experience in the field of Financial Accounts, finance and, business studies, thereby looking forward to sharing those experiences in such a way that finds suitable solutions to your query.
Thus, please feel free to contact me regarding the same.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
You place the substance A(g) in a container. Consider the following reaction under standard conditions to produce the substance B(g): For this reaction as written, the equilibrium constant is a very...
-
In this problem you need to draw two pictures of solutions in beakers at different points in time. Time zero (t = 0) will be the hypothetical instant at which the reactants dissolve in the solution...
-
Suppose you have a subject property with a 105,000 sq. ft. lot and existing improvements for which you estimate the reproduction cost new to be $2,500,000, physical deterioration to be $400,000,...
-
A company uses exponential smoothing with trend to forecast monthly sales of its product, which show a trend pattern. At the end of week 5, the company wants to forecast sales for week 6. The trend...
-
Zillow.com is an online database that provides real estate information for U.S. homes that are for rent or sale. It also presents statistics on recently sold homes. The following table gives various...
-
The two-dimensional velocity field for an incompressible Newtonian fluid is described by the relationship \[ \mathbf{V}=\left(12 x y^{2}-6 x^{3} ight) \hat{\mathbf{i}}+\left(18 x^{2} y-4 y^{3} ight)...
-
Aquatran Incorporated uses leases as a method of selling its products. In early 2011, Aquatran completed construction of a passenger ferry for use between Manhattan and Staten Island. On April 1,...
-
If individual values that underline ethics are developed at a young age, what might this suggest about the potential for ethical conflicts to arise within an organization?
-
The cancer drug cisplatin, Pt(NH 3 ) 2 Cl 2 , can be made by reacting (NH 4 ) 2 PtCl 4 with ammonia in aqueous solution. Besides cisplatin, the other product is NH 4 Cl. (a) Write a balanced equation...
-
One half liter (500. mL) of 2.50 M HCl is mixed with 250. mL of 3.75 M HCl. Assuming the total solution volume after mixing is 750. mL, what is the concentration of hydrochloric acid in the resulting...
-
Tom Adams has received a job offer from a large investment bank as a clerk to an associate banker. His base salary will be $ 55,000. He will receive his first annual salary payment one year from the...
-
Magen's Bakery, Inc., is considering the purchase of a new cake mixing equipment for $200,000. This would result in an increase in earnings before interest and taxes of $50,000 per year. To operate...
-
2. a. If you lift the shopping bag of 5 kg by hand by bending the elbow only, calculate the torque at the elbow point if the distance from elbow to hand is 30.0 cm
-
16. Your cousin makes a parallel plate capacitor out of two square metal plates of edge length l = 25.4 mm and a nonconducting glass plate of the same area, thickness d = 2.50 mm, and dielectric...
-
An extension cord that has a length of 8.35m8.35m is made from wire with a resistivity of 8.74108m and a diameter of 0.882cm. What is the resistance, in ohms, of the extension cord? If a current of...
-
Diana is 62 years old and would like to retire in two years. How will her retirement benefits be affected if she decides to move forward with her retirement plan?
-
How does authorized stock differ from outstanding stock?
-
Smthe Co. makes furniture. The following data are taken from its production plans for the year. Required: 1. Determine the hazardous waste disposal cost per unit for chairs and for tables if costs...
-
The following mass spectrum is for octane. a) Which peak represents the molecular ion? b) Which peak is the base peak? c) Draw the structure of the fragment that produces the base peak. 100 80 60 40...
-
Calculate the HDI for each molecular formula. a) C 4 H 6 b) C 5 H 8 c) C 40 H 78 d) C 72 H 74 e) C 6 H 6 O 2 f) C 7 H 9 NO 2 g) C 8 H 10 N 2 O h) C 5 H 7 Cl 3 i) C 6 H 5 Br j) C 6 H 12 O 6
-
Following are the IR spectrum and mass spectrum of an unknown compound. Propose at least two possible structures for the unknown compound. 100- 80- 40- 20- 4000 3500 3000 2500 2000 1500 1000 500...
-
Summary of baked items sold monthly is mentioned below: Item Sales volume Sale price Cookies- 100 $7 Cupcakes- 100 $6 Muffins- 80 $8 Other N/A $600 (monthly total) Janny has projected monthly fixed...
-
Mary received a chat message from her "boss" saying that his user ID and password have expired and he is not able to login to company network to continue with his work. He requests Mary to provide...
-
Fie Co. is an engineering company that works in the Energy sector and an oil and gas producer in Turkey. On 21 April 2019, this company entered a contract with Alwand Co. which is from health care...

Study smarter with the SolutionInn App


