The glycinate ion, H 2 NCH 2 CO 2 , formed by deprotonation of the amino
Question:
The glycinate ion, H2NCH2CO2−, formed by deprotonation of the amino acid glycine, can function as a bidentate ligand, coordinating to a metal through the nitrogen of the amino group and one of the oxygen atoms.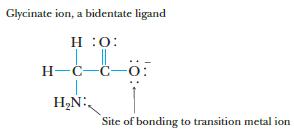
A copper complex of this ligand has the formula Cu(H2NCH2CO2)2(H2O)2. For this complex, determine the following:
(a) The oxidation state of copper
(b) The coordination number of copper
(c) The number of unpaired electrons
(d) Whether the complex is diamagnetic or paramagnetic
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





